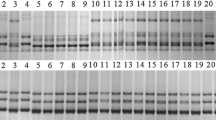
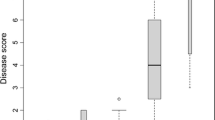
Abstract
We have previously demonstrated that in the diploid rose population 97/9 resistance to the powdery mildew race 9 is controlled by a major dominant resistance gene, Rpp1. In the study reported here, we isolated several molecular markers closely linked to Rpp1 via bulked segregant analysis, with the gene being tagged in an interval of 5 cM between the two most adjacent markers. It was possible to convert the most closely linked amplified fragment length polymorphic (AFLP) marker into a sequence-characterised amplified region (SCAR) segregating in the same manner. Indirect mapping of Rpp1 in relation to the black spot resistance gene Rdr1 revealed no linkage between the two R genes. Furthermore, the genetic model based on a single dominant resistance gene was supported by the marker data.



Similar content being viewed by others
References
Bevan JR, Crute IR, Clarke DD (1993) Variation for virulence in Erysiphe fischeri from Senecio vulgaris. Plant Pathol 42:622–635
Braun U, Takamatsu S (2000) Phylogeny of Erysiphe, Microsphaera, Uncinula (Erysipheae) and Cystotheca, Podosphaera, Sphaerotheca (Cystotheceae) inferred from rDNA ITS sequences—some taxonomic consequences. Schlechtendalia 4:1–33
Braun PW, Turgut I (1995) The virulence structure of mildew populations on wild barley in Turkey. J Plant Dis Prot 102:593–598
Chaanin A (2003) Breeding/selection strategies for cut roses. In: Roberts AV, Debener T, Gudin S (eds) Encyclopedia of rose science. Elsevier, Oxford, pp 33–41
Debener T, Mattiesch L (1999) Construction of a genetic linkage map for roses using RAPD and AFLP markers. Theor Appl Genet 99:891–899
Fried PM, MacKenzie DR, Nelson RR (1981) Yield loss caused by Erysiphe graminis f. sp. tritici on single culms of ‘Chancellor’ wheat and four multilines. Z Pflanzenk Pflanzen 88:256–264
Hattendorf A, Linde M, Kaufmann H, Mattiesch L, Debener T (2004) Genetic analysis of rose resistance genes and their localisation in the rose genome. Acta Hortic (in press)
Horst RK (1983) Compendium of rose diseases. American Phytopathological Society, St. Paul
Huang XQ, Hsam SLK, Zeller FJ (1997) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em. Thell.). 4. Gene Pm24 in Chinese landrace Chiyacao. Theor Appl Genet 95:950–953
Huang CC, Cui YY, Weng CR, Zabel P, Lindhout P (2000) Development of diagnostic PCR markers closely linked to the tomato powdery mildew resistance gene Ol-1 on chromosome 6 of tomato. Theor Appl Genet 101:918–924
Jones JDG (2001) Putting knowledge on disease resistance genes to work. Curr Opin Plant Biol 4:281–287
Kaufmann H, Mattiesch L, Lörz H, Debener T (2003) Construction of a bac library of Rosa rugosa Thunb. and assembly of a contig spanning Rdr1, a gene that confers resistance to black spot. Mol Genet Genomics 268:666–674
Keller B, Feuillet C, Messmer M (2000) Genetics of disease resistance: basis concepts and application in resistance breeding. In: Slusarenko AJ, Fraser RSS, Van Loon LC (eds) Mechanisms of resistance to plant diseases. Kluwer, Dordrecht, pp 101–136
Kobayashi N, Horikoshi T, Katsuyama H, Handa T, Takayanagi K (1998) A simple and efficient DNA extraction method for plants, especially woody plants. Plant Tissue Cult Biotechnol 4:77–81
Kunoh H (1995) Host-parasite specificity in powdery mildews. In: Singh US, Kohmoto K, Singh RP (eds) Pathogenesis and host specificity in plant diseases: histopathological, biochemical, genetic and molecular bases, vol. 2 Eukaryotes. Elsevier, Oxford, pp 239–250
Linde M, Debener T (2003) Isolation and identification of eight races of powdery mildew of roses [Podosphaera pannosa (Wallr.: Fr.) de Bary] and the genetic analysis of the resistance gene Rpp1. Theor Appl Genet 107:256–262
Liu J, Liu D, Tao W, Li W, Wang S, Chen P, Cheng S, Gao D (2000) Molecular marker-facilitated pyramiding of different genes for powdery mildew resistance in wheat. Plant Breed 119:21–24
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential and durable resistance. Annu Rev Phytopathol 40:349–379
Noack R (2003) Breeding/selection strategies for disease and pest resistance. In: Roberts AV, Debener T, Gudin S (eds) Encyclopedia of rose science. Elsevier, Oxford, pp 49–55
Paran I, Michelmore RW (1993) Development of reliable PCR-based markers linked to downy mildew resistance genes in lettuce. Theor Appl Genet 85:985–993
Paterson AH (1996) Physical mapping and map-based cloning: bridging the gap between DNA markers and genes. In: Paterson AH (ed) Genome mapping in plants. Academic, San Diego, pp 55–61
Reimann-Philipp (1981) Cytogenetics and breeding in diploid roses from the triploid hybrid R. multiflora × garden cultivars. In Eucarpia (ed): Proc Eucarpia Ornamentals Meet Rose Breed. Eucarpia, pp 27–29
Rommens CM, Kishore GM (2000) Exploiting the full potential of disease-resistance genes for agricultural use. Curr Opin Biotechnol 11:120–125
Rong JK, Millet E, Manisterski J, Feldmann M (2000) A new powdery mildew resistance gene: introgression from wild emmer into common wheat and RFLP-based mapping. Euphytica 115:121–126
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor
Schaffer JF, Roelfs AP (1985) Estimated relation between numbers of urediniospores of Puccinia-graminis f. sp. tritici and rates of occurrence of virulence. Phytopathology 75:749–750
Schmidt R, West J, Love K, Lenehan Z, Lister C, Thompson H, Bouchez D, Dean C (1995) Physical map and organization of Arabidopsis thaliana chromosome 4. Science 270:480–483
Schneider KA, Brothers ME, Kelly JD (1997) Marker-assisted selection to improve drought resistance in common bean. Crop Sci 37:51–60
Schönfeld M, Ragni A, Fischbeck G, Jahoor A (1996) RFLP mapping of three new loci for resistance genes to powdery mildew (Erysiphe graminis f. sp. hordei) in barley. Theor Appl Genet 93:48–56
Stam P, Van Ooijen JW (1995) joinmap version 2.0: software for the calculation of genetic linkage maps. CPRO-DLO, Wageningen
Tanksley SD, Young ND, Paterson AH, Bonierbale MW (1989) RFLP mapping in plant breeding: new tools for an old science. Biotechnology 7:257–264
Tiwari KR, Penner GA, Warkentin TD (1998) Identification of coupling and repulsion phase RAPD markers for powdery mildew resistance gene er-1 in pea. Genome 41:440–444
Urbanietz A (2002) Genetische und molekulare Charakterisierung der Resistenz des Apfels gegen den Echten Mehltau und der Virulenz des Erregers Podosphaera leucotricha (Ell. Et Ev.) Salm. PhD thesis, University of Hannover, Germany
Van der Beek JG, Pet G, Lindhout P (1994) Resistance to powdery mildew (Oidium lycopersicon) in Lycopersicon-hirsutum is controlled by an incompletely dominant gene Ol-1 on chromosome 6. Theor Appl Genet 89:467–473
Von Malek B, Weber WE, Debener T (2000) Identification of molecular markers linked to Rdr1, a gene conferring resistance to black spot in roses. Theor Appl Genet 101:977–983
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Wolfe MS (1985) The current status and prospects of multiline cultivars and variety mixtures for disease resistance. Annu Rev Phytopathol 23:251–273
Wolfe MS, McDermott JM (1994) Population genetics of plant pathogen interactions: the example of the Erysiphe graminis–Hordeum vulgare pathosystem. Annu Rev Phytopathol 32:89–113
Zeller FJ, Kong L, Hartl L, Mohler V, Hsam SLK (2002) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.) 7. Gene Pm29 in line Pova. Euphytica 123:187–194
Acknowledgements
This project was supported by a grant from the Ministry of Economics, Technology and Transport of the Land Schleswig-Holstein and by the companies W. Kordes Söhne (Germany) and Rosen Tantau (Germany). We thank K. Sabin for valuable technical assistance and Prof. A.V. Roberts (UEL, London) for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Nybom
Rights and permissions
About this article
Cite this article
Linde, M., Mattiesch, L. & Debener, T. Rpp1, a dominant gene providing race-specific resistance to rose powdery mildew (Podosphaera pannosa): molecular mapping, SCAR development and confirmation of disease resistance data. Theor Appl Genet 109, 1261–1266 (2004). https://doi.org/10.1007/s00122-004-1735-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-004-1735-4