Abstract
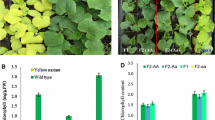
Cytoplasmic line 2 (CL2) has been previously reported as a cytoplasmically inherited chlorophyll-deficient mutant selected from a chloroplast-mutator genotype of barley. It was characterized by a localized effect on the upper part of the first-leaf blade. At emergence the CL2 seedlings-phenotype varied from a grainy light green to an albino color. They gradually greened during the following days, starting from the base of the blade and extending to cover most of its surface when it was fully grown. The present results, from both light microscopy and transmission electron microscopy (TEM), confirmed the previously described positional and time-dependent expression of the CL2 syndrome along the first-leaf blade. During the first days after emergence, light microscopy showed a normally developed chloroplast at the middle part of the CL2 first-leaf blade, meanwhile at the tip only small plastids were observed. TEM showed that the shapes and the internal structure of the small plastids were abnormal, presenting features of proplastids, amyloplasts and/or senescent gerontoplasts. Besides, they lack plastid ribosomes, contrasting with what was observed inside chloroplasts from normal tips, which presented abundant ribosomes. Phenotypic observations and spectrophotometric analysis of seedlings produced by mother plants that had been grown under different temperatures indicated that higher temperatures during seed formation were negatively associated with pigment content in CL2 seedlings. In contrast, higher temperatures during the growth of CL2 seedlings have been associated with increased pigment content. Aqueous solution with kanamycin and streptomycin, which are antibiotics known to interfere with plastid gene translation, were used for imbibition of wild-type and CL2 seeds. Antibiotic treatments differentially reduced the chlorophyll content in the upper part of the first-leaf blade in CL2, but not in wild-type seedlings. These results suggest that in the wild-type, plastid-gene proteins which are necessary for chloroplast development and chlorophyll synthesis in the upper part of the first-leaf blade are usually synthesized during embryogenesis. However, under certain circumstances, in CL2 seedlings, they would be synthesized after germination. In addition, a shortening of the sheath has been observed in association with pigment decrease suggesting the existence of plastid factors affecting the expression of some nuclear genes. We consider the CL2 mutant a unique experimental material useful to study biological phenomena and external factors regulating plastid, and nuclear gene expression during embryogenesis and early seedling development.








Similar content being viewed by others
References
Baumgartner BJ, Rapp JC, Mullet JE (1989) Plastid transcription activity and DNA copy number increase early in Barley chloroplast development. Plant Physiol 89:1011–1018
Börner T (1986) Chloroplast control of nuclear gene function. Endocyt C Res 3:265–274
Börner T, Sears B (1986). Plastome mutants. Plant Mol Biol Rep 4:69–72
Bradbeer JW, Atkinson YE, Börner T, Hagemann R (1979) Cytoplasmic synthesis of plastid polypeptides may be controlled by plastid-synthesised RNA. Nature 279:816–817
Bruick RK, Mayfield SP (1999) Light-activated translation of chloroplast mRNAs. Trends Plant Sci 4:190–195
Churin Y, Hess W, Börner T (1999) Cloning and characterization of three cDNAs encoding chloroplast RNA-binding proteins from barley (Hordeum vulgare L.): differential regulation of expression by light and plastid development. Curr Genet 36:173–181
Green BR, Salter H (1996) Light regulation of nuclear-encoded thylakoid proteins. In: Frontiers in Molecular Biology Series, Oxford University Press, pp 75–103
Gustafsson Å (1942) The plastid development in various types of chlorophyll mutations. Hereditas 28:483–492
Hess WR, Börner T (1999) Organellar RNA polymerases of higher plants. Int Rev Cytol 190:1–59
Hess WR, Müller A, Nagy F, Börner T (1994) Ribosome-deficient plastids affect transcription of light-induced nuclear genes: genetic evidence for a plastid-derived signal. Mol Gen Genet 242:305–312
Hess WR, Linke B, Börner T (1997) Impact of plastid differentiation on transcription of nuclear and mitochondrial genes. In: Schenk et al. (ed) Eukaryotism and symbiosis, pp 233–242
Krupinska K, Apel K (1989) Light-induced transformation of etioplasts to chloroplasts of barley without transcriptional control of plastid gene expression. Mol Gen Genet 219:467–473
Langdale JA, Nelson T (1991) Spatial regulation of photosynthetic development in C4 plants. Science 7:191–196
Leon P, Arroyo A, Mackenzie S (1998) Nuclear control of plastid and mitochondrial development in higher plants. Ann Rev Plant Physiol Plant Mol Biol 49:453–480
Mache R, Zhan DX, Lerbs-Mache S, Harra KH, Villain P, Gauvincs S (1997) Nuclear control of early plastid differentiation. Plant Physiol Biochem 35:199–203
Maclachlan S, Zalik S (1963) Plastid structure, chlorophyll concentration and free amino-acid composition of a chlorophyll mutant of barley. Can J Bot 41:1053–1062
Mayfield SP, Cohen A (1998) Translational regulation in the chloroplasts. In: Bailey-Serres J, Gallic DR (eds) A look beyond transcription. Am Soc Plant Physiol, pp 174–179
Mullet J (1988) Chloroplast development and gene expression. Annu Rev Plant Physiol Plant Mol Biol 39:475–502
Mullet J (1993) Dynamic regulation of chloroplast transcription. Plant Physiol 103:309–313
Myhill RR, Konzak CF (1967) A new technique for culturing and measuring barley seedlings. Crop Sci 7:275–277
Oelmüller R, Levitan I, Bergfeld R, Rajasek VK, Mohr H (1986) Expression of nuclear genes as affected by treatments acting on the plastids. Planta 168:482–492
Prina AR (1992) A mutator nuclear gene inducing a wide spectrum of cytoplasmically inherited chlorophyll deficiencies in barley. Theor Appl Genet 85:245–251
Prina AR (1996) Mutator induced cytoplasmic mutants in barley: genetic evidence of activation of a putative chloroplast transposon. J Hered 87:385–389
Rodermel S (2001) Pathways of plastid-to-nucleus signaling. Trends Plant Sci 6:471–478
Stern DB, Higgs DC, Yang J (1997) Transcription and translation in chloroplasts. Trends Plant Sci 2:308–315
Somanchi A, Mayfield SP (1999) Nuclear-chloroplast signalling. Curr Opin Plant Biol 2:404–409
Taylor WC (1989) Regulatory interactions between nuclear and plastid genomes. Annu Rev Plant Physiol Plant Mol Biol 40:211–233
Taylor WC, Barkan A, Martienssen RA (1987) Use of nuclear mutants in the analysis of chloroplast development. Dev Genet 8:305–320
Wettstein D von (1961) Nuclear and cytoplasmic factors in development of chloroplast structure and function. Can J Bot 39:1537–1545
Wettstein D von, Gough S, Gamini Kannangara C (1995) Chlorophyll biosynthesis. Plant Cell 7:1039–1067
Acknowledgements
The authors thank Prof. Barbara Sears (Michigan State University, USA) for critical reading of the manuscript, Mrs. Edith García, Mr. Abel Moglie, Mr. Roberto Civitillo and Mr. Miguel Díaz for their skilfull technical assistance. They also thank the financial support from CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina), PIP 96/4414 and FONCYT- SeCyT (Fondo Nacional para la Ciencia y la Tecnología-Secretaría de Ciencia, Tecnología e Innovación Productiva, Argentina), PICT 08/04841.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hagemann
Rights and permissions
About this article
Cite this article
Prina, A.R., Arias, M.C., Lainez, V. et al. A cytoplasmically inherited mutant controlling early chloroplast development in barley seedlings. Theor Appl Genet 107, 1410–1418 (2003). https://doi.org/10.1007/s00122-003-1391-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-003-1391-0