Abstract
Wolbachia is a maternally inherited bacterium that manipulates the reproduction of its host. Recent studies have shown that male-killing strains can induce cytoplasmic incompatibility (CI) when introgressed into a resistant host. Phylogenetic studies suggest that transitions between CI and other Wolbachia phenotypes have also occurred frequently, raising the possibility that latent CI may be widespread among Wolbachia. Here, we investigate whether a parthenogenesis-inducing Wolbachia strain can also induce CI. Parthenogenetic females of the parasitoid wasp Asobara japonica regularly produce a small number of males that may be either infected or not. Uninfected males were further obtained through removal of the Wolbachia using antibiotics and from a naturally uninfected strain. Uninfected females that had mated with infected males produced a slightly, but significantly more male-biased sex ratio than uninfected females that had mated with uninfected males. This effect was strongest in females that mated with males that had a relatively high Wolbachia titer. Quantitative PCR indicated that infected males did not show higher ratios of nuclear versus mitochondrial DNA content. Wolbachia therefore does not cause diploidization of cells in infected males. While these results are consistent with CI, other alternatives such as production of abnormal sperm by infected males cannot be completely ruled out. Overall, the effect was very small (9%), suggesting that if CI is involved it may have degenerated through the accumulation of mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wolbachia is a cytoplasmically inherited bacterium, known for its ability to manipulate reproduction in its arthropod hosts (Stouthamer et al. 1999). These manipulations include the induction of parthenogenesis (PI), male killing, feminization, and cytoplasmic incompatibility (CI). The first three result in highly female-biased sex ratios while CI decreases the offspring production of uninfected females by inducing sterility in crosses between infected males and uninfected females. In haplodiploids, only fertilized eggs, which normally develop into diploid females, suffer CI while unfertilized eggs, which develop into haploid males, do not. Incompatible eggs either die or are converted into males. In both cases, CI results in a more male-biased sex ratio.
Phylogenetically, Wolbachia strains inducing different phenotypes do not form monophyletic clusters (Baldo et al. 2006), suggesting that switches between phenotypes have occurred repeatedly. Recurrent acquisition and loss of genes involved in reproductive manipulations in Wolbachia genomes may lead to rapid changes in the phenotype. Alternatively, all manipulations may be induced by the same Wolbachia strain, but their expression may depend on the host genetic background. In support of the latter, several recent studies have shown that some Wolbachia can switch rapidly between male killing and CI (e.g., Jaenike 2007; Hornett et al. 2008). For example, Hornett et al. (2008) showed that a male-killing Wolbachia infecting the butterfly Hypolimnas bolina induces CI when introgressed into host strains that are resistant to male killing, showing it has a latent ability to induce CI. Whether Wolbachia that normally induce parthenogenesis or feminization also have the ability to induce CI is unknown.
Hurst et al. (2002) showed theoretically that CI-only strains are highly susceptible to invasion by mutants that can manipulate host sex ratios while retaining their CI ability. When sex ratio distortion is complete (i.e., infected females produce no males), as is common for parthenogenesis-inducing strains, CI is not expressed anymore, and the ability to cause CI is expected to degrade by selection and/or mutation, resulting in a strain that only distorts the sex ratio (Hurst et al. 2002).
In this study, we investigate whether the Wolbachia strain wAjap that normally induces parthenogenesis in its Drosophila parasitoid host Asobara japonica (Hymenoptera: Braconidae) can also induce CI. Phylogenetic analysis showed wAjap to be closely related to several strains that induce CI (Kremer et al. 2009), suggesting that transitions between CI and other phenotypes have occurred in this group. Populations of A. japonica on the main islands of Japan reproduce through Wolbachia-induced thelytokous parthenogenesis, in which females are produced from unfertilized eggs. Populations from the southern subtropical islands are uninfected and reproduce through arrhenotoky, in which females develop from fertilized, diploid eggs and males from unfertilized, haploid eggs (Mitsui et al. 2007; Kremer et al. 2009). During routine culturing, infected A. japonica females regularly produce small numbers of males (rarely many), some of which are infected with Wolbachia, a situation rarely observed in species infected with PI Wolbachia. This allowed us to investigate whether wAjap is able to induce CI.
Materials and methods
Asobara strains and antibiotic treatment
A. japonica strains were kindly provided by M. T. Kimura from cultures derived from the field samples described in Mitsui et al. (2007). An infected, thelytokous population from Kagoshima and an uninfected, arrhenotokous population from Amami-Oshima were used in all experiments. Culturing and removal of Wolbachia were described in Kremer et al. (2009). Briefly, parasitoid eggs and larvae were exposed to antibiotics through the host’s hemolymph. Most of the antibiotic-treated larvae developed as aposymbiotic females. These females produced only males when allowed to oviposit, indicating they were cured of their Wolbachia infection. Few antibiotic-treated larvae developed directly as aposymbiotic males. Hence, we used both males from the first generation, which had been exposed to the antibiotic, and from the second generation, of which only their mother had been exposed.
Mating experiments
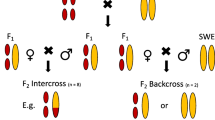
To obtain virgin females from the arrhenotokous strain, pupae were transferred individually to polymerase chain reaction (PCR) tubes just prior to emergence. These tubes were checked twice daily for emerged females, which were then transferred to a small glass tube (2.5 × 8.0 cm) containing a layer of agar. Each female was provided with a single male of one of five types (arrhenotokous and thelytokous refer to the line of origin): uninfected arrhenotokous, uninfected thelytokous antibiotic-exposed, uninfected thelytokous from antibiotic-exposed mother, naturally uninfected thelytokous, and infected thelytokous. Males develop faster than females. To ensure that the males were virgin, the culture jars were checked every 3 h around the time of emergence and virgin males were transferred to fresh jars and kept in single-sex groups. No males were collected from jars in which females had started emerging. The mating pair was kept together in the glass tube for 2 days, after which the female was allowed to parasitize about 200 Drosophila melanogaster larvae. All males were checked for Wolbachia infection through PCR assay (see below). The numbers of male and female offspring emerging from these crosses were scored for the next 4 weeks.
DNA extraction
DNA extractions were performed using the DNeasy Blood & Tissue Kit (Qiagen) according to the manufacturer’s protocol, using mini spin columns. Before starting the DNA extraction, each wasp was transferred to a new 1.5-ml Eppendorf tube. After evaporation of remaining ethanol, tissue lysis buffer (ATL) was added to the tube and the wasp was crushed using a plastic pestle. The tissue was incubated overnight in proteinase K at 56°C. The DNA was dissolved in 100 μl elution buffer (AE).
Wolbachia PCR assay
Wasps were tested for Wolbachia infection by amplifying the Wolbachia-specific wsp gene, with primers wsp81F and wsp691R (Braig et al. 1998; Zhou et al. 1998) and the Wolbachia-specific ftsZ gene (Holden et al. 1993, Sinkins et al. 1995). PCRs for both genes were performed in a total volume of 20.0 μl, containing 1× PCR buffer (Qiagen), 62.5 μM dNTPs, 1 U Taq polymerase, 250 nM forward primer, 250 nM reverse primer, and 1.0 μl DNA template. A PTC-200 DNA Engine Thermal Cycler PCR machine (MJ Research) was used for all PCRs. PCR conditions for the wsp gene were as follows: 3 min at 94°C, then 35 cycles of 1 min at 94°C; 1 min at 55°C and 1 min at 72°C; and finally 5 min at 72°C. PCR conditions for the ftsZ gene were as follows: 3 min at 94°C, then 35 cycles of 45 s at 94°C; 1 min at 55°C and 1 min at 72°C; and finally 5 min at 72°C. All PCR products were run on a 2% agarose gel and visualized using ethidium bromide staining.
Real-time quantitative PCR
To test whether the sex ratios induced by infected males were affected by Wolbachia density, we conducted real-time quantitative PCR (qPCR) on the DNA samples from the mating experiment. We also compared Wolbachia density between infected males and infected females. Wolbachia density was assessed by quantifying the copy number of the Wolbachia-specific wsp gene, using the nuclear 18S gene to control for DNA concentration. The number of Wolbachia cells was determined by using the generalist primers 81F/691R which amplified the single-copy wsp gene as described in Mouton et al. (2004). The multicopy nuclear gene 18SrRNA was amplified using the primers 18s.lo1/NS58+2, as described in Mouton et al. (2009).
Furthermore, we tested whether Wolbachia infection resulted in diploidization of cells in males. We again quantified the nuclear 18S gene and compared this to the quantity of the mitochondrial COI (cytochrome oxidase I subunit) gene, which should not be affected by diploidization. In this test, we compared infected with uninfected males. We designed new primers from the alignment of the COI sequences of 16 A. japonica strains (Murata et al. 2009) especially to optimize qPCR reactions: AjapCOI-159F (5′-ACCTGTAATATTAGGTGGATTTGG-3′) and AjapCOI-289R (5′-CCAACACCTACATTTAATATTCCTCT-3′; amplified product 139 bp). The PCR conditions consisted of 10 min at 95°C followed by 35 cycles, each consisting of denaturing for 10 s at 95°C, annealing for 10 s at 54°C, and elongation for 10 s at 72°C. qPCR reactions were performed on the LightCycler® 480 Real-Time PCR System (Roche, France). The 10 μL reaction mix contained 200 nM of each primer, 5 μL of LightCycler® 480 SYBR Green I Master (Roche, France), and 1 μL of template DNA.
MLST sequencing
In order to assess whether the Wolbachia infecting the male A. japonica was indeed wAjap (and not an additional strain that had escaped detection in infected females), we sequenced a set of five MLST genes (multilocus sequence typing) and the wsp gene for one infected male and one infected female for each of five thelytokous A. japonica strains (Kagoshima, Sapporo, Hirosaki, Sendai, and Tokyo; Mitsui et al. 2007). The protocols for PCR and sequencing were described in Baldo et al. (2006).
Statistical analysis
The sex ratios of the offspring produced by females mated to different types of males were compared using generalized linear models (glm) with a binomial error distribution and an empirically estimated scale parameter. The number of males was the response variable and the total number of offspring the binomial denominator. Significance was assessed by removing explanatory variables from the model and comparing the change in deviance using an F test. Clutch size was compared using ANOVA. Since the data sets for the qPCR experiments did not follow normal distributions (Shapiro test), we used nonparametric Wilcoxon rank sum tests, with an alpha of 0.05. All analyses were performed in R 2.9.2 (R Development Core Team 2009), except the power analysis, for which we used the sample size calculator available at http://www.stat.ubc.ca/~rollin/stats/ssize/n2.html.
Results
Mating experiments
A total of 34 males were produced by infected mothers, of which 18 (53%) tested positive for Wolbachia in the PCR assay. The sex ratios produced by arrhenotokous females differed significantly, depending on the type of male they had mated with (Fig. 1; glm F 4,64 = 2.81, P = 0.03). Lumping the uninfected male groups did not result in a significant change in deviance of the model (comparison of the model with five male types (df = 4) to a model with only infection status (df = 1): glm F 3,64 = 0.31, P = 0.82). However, the infection status of the male had a significant effect on the sex ratio of the offspring (glm F 1,67 = 10.61, P = 0.002). The difference in sex ratio between infected and uninfected groups was 9% (infected, 37% male offspring; uninfected, 28% male offspring). Clutch size was highly variable, but did not differ between females that mated with infected or uninfected males (infected males, mean = 114.39 ± 24.91 SD; uninfected males, mean = 104.96 ± 33.91 SD; F 1,67 = 1.16, P = 0.28). However, the variability meant that we would have needed to sample about 100 females in each group to detect a 9% difference in clutch size (one-sided test, alpha = 0.05, power = 0.8).
Wolbachia density
Wolbachia density was significantly lower in infected males than in infected females (ratio wsp/18S, nmales = 18, nfemales = 20, Wilcoxon W = 360, P = 0.0000001). Among infected males, Wolbachia density correlated with the sex ratio produced by the females they mated with (Fig. 2; ratio wsp/18S glm F1,16 = 10.82, P = 0.005). This correlation was also significant when COI was used instead of 18S to control for DNA content (ratio wsp/COI glm F1,15 = 8.53, P = 0.01). Thus, males with higher Wolbachia densities induced more male-biased clutches than males with lower Wolbachia titers. There was no difference in the 18S/COI ratio between infected and uninfected males from the thelytokous strain, indicating that Wolbachia does not cause diploidization of cells in infected males (ninfected = 17, nunifected = 13, Wilcoxon W = 111, P = 0.99).
MLST sequencing
The sequences of Wolbachia genes obtained from all males and all females of all strains were identical (GenBank accession numbers HM241181–HM241186). There was thus no indication that strains other than wAjap are infecting A. japonica or that infected thelytokous males harbor a different Wolbachia strain than the one that is normally inducing parthenogenesis in this species.
Discussion
Our results show a significant difference between the sex ratios produced by females that were mated with Wolbachia-infected males and those of females that were mated to uninfected males. This effect was strongest when the Wolbachia density in the infected male was relatively high. Uninfected thelytokous males did not show a reduction in fertilization capacity compared to arrhenotokous males, indicating normal spermatogenesis and absence of nuclear incompatibilities between individuals originating from thelytokous and arrhenotokous populations. Furthermore, Wolbachia did not cause (partial) diploidization of infected males. No other symbiont has been detected in A. japonica (Kremer et al. 2009), and MLST indicated that the Wolbachia strain that caused this effect was the same as the strain that induces parthenogenesis in the females. Wolbachia infection in males thus causes male-biased sex ratios when crossed with uninfected females.
Different hypotheses can be proposed for explaining this pattern. First, infected males could be mosaics of haploid and diploid cells as happens in Nasonia (Kamping et al. 2007), leading to production of some diploid sperm. However, infected males did not show an elevated ratio of nuclear versus mitochondrial DNA content compared to uninfected males as would be expected under diploidization. Furthermore, diploid sperm should lead to an increase in mortality in the offspring of these males, for which we find no evidence. Second, the presence of Wolbachia may result in a reduction in sperm production, leading to the observed pattern. However, this reduction needs to be substantial because males used in this experiment were virgin and only had one female to mate with. The third hypothesis is that the parthenogenesis-inducing Wolbachia strain wAjap is able to induce CI when present in males. One way to test for this hypothesis would be to test for the rescue ability of wAjap in infected females. Unfortunately, females from thelytokous strains are not receptive to mating (Kremer et al. 2009), making this experiment impossible.
The mean difference in sex ratio induced by infected versus uninfected males was very small (9%) and the frequency distributions between the groups overlapped. If this effect is indeed due to CI expression, there are several potential explanations for the low degree of CI induced by the normally parthenogenesis-inducing wAjap. We show that the sex ratio produced by females is correlated to the Wolbachia titer in their mates. The males with the highest Wolbachia titers in our sample induced sex ratios up to 98% males, which approach the sex ratio bias seen in incompatible crosses in other hymenoptera infected with CI Wolbachia. For example, the difference in sex ratio between compatible and incompatible crosses was 35% in Asobara tabida (Dedeine et al. 2004) and 40% in Trichopria cf. Drosophilae (Vavre et al. 2002). CI results in all-male broods in Nasonia vitripennis and Leptopilina heterotoma (Bordenstein et al. 2003; Vavre et al. 2001, respectively). Thus, wAjap may be able to induce full CI, but the overall low degree of CI that we saw may be due to low Wolbachia titers in most infected males. However, given that only one mating resulted in nearly complete sex bias, it is too early to rule out alternative explanations. A. japonica may be a host in which Wolbachia is not able to induce strong CI or A. japonica may be a competent host, but wAjap may not be able to induce strong CI. Hurst et al. (2002) predicted that an ancestral ability to induce CI would degrade when an invading mutant that distorts sex ratio approaches 100% efficiency. As far as is known, the infection with wAjap is fixed on the islands where it occurs. wAjap normally induces >97% parthenogenesis. In the field, infected males cannot encounter uninfected females, since infected and uninfected populations are on different islands, and females from thelytokous populations do not elicit courtship by males. CI is thus not expressed in the field. In addition, any fitness cost imposed by the retention of CI ability will select for its loss. It is possible that the low level of CI induced by wAjap is partly due to this ability having degenerated by selection and/or neutral mutation accumulation.
Contrary to the situation observed in A. japonica, several male-killing Wolbachia induce complete CI when introgressed within a resistant host background. Why is low CI not seen in male-killing Wolbachia? The first possible explanation is related to bacterial load. Resistance to male killing does not rely on the reduction of the Wolbachia titer, but to a direct resistance to the male-killing phenotype. On the contrary, we show here that infected males have low Wolbachia density in A. japonica, and this might be related to the fact that individuals with high density are diploidized and thus converted into females. This difference in the mechanisms leading to male production may structurally impose lower CI in PI Wolbachia compared to male-killing Wolbachia. The other possibility is related to the dynamics of the co-evolutionary process between partners. Male killing imposes strong selection for suppression in its host because it kills a large proportion of offspring (Hurst et al. 2002). Thus, hosts infected by a male-killing Wolbachia may evolve resistance before sufficient time has passed to allow degradation of its ancestral CI ability (Hornett et al. 2008). In contrast, parthenogenesis is a very efficient mode of reproduction and selection for its suppression may be weak. In particular, as soon as parthenogenesis is fixed, as seems to be the case in A. japonica, there is no selection for resistance. Further understanding of the interplay between parthenogenesis and CI induction by Wolbachia might come from comparative studies. If the CI ability has indeed degraded in wAjap, it might be possible to find stronger CI in host species that have only recently become infected with a parthenogenesis-inducing Wolbachia.
References
Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudbury RR, Hayashi C, Maiden MCJ, Tettelin H, Werren JH (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110
Bordenstein SR, Uy JJ, Werren JH (2003) Host genotype determines cytoplasmic incompatibility type in the haplodiploid genus Nasonia. Genetics 164:223–233
Braig HR, Zhou W, Dobson SL, O’Neill SL (1998) Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol 180:2373–2378
Dedeine F, Vavre F, Shoemaker DD, Bouletreau M (2004) Intraindividual coexistence of a Wolbachia strain required for host oogenesis with two strains inducing cytoplasmic incompatibility in the wasp Asobara tabida. Evolution 58:2167–2174
Holden PR, Brookfield JFY, Jones P (1993) Cloning and characterization of an ftsZ homologue from a bacterial symbiont of Drosophila melanogaster. Mol Gen Genet 240:213–220
Hornett EA, Duplouy AMR, Davies N, Roderick GK, Wedell N, Hurst GDD, Charlat S (2008) You can’t keep a good parasite down: evolution of a male-killer suppressor uncovers cytoplasmic incompatibility. Evolution 62:1258–1263
Hurst GDD, Jiggins FM, Pomiankowski A (2002) Which way to manipulate host reproduction? Wolbachia that cause cytoplasmic incompatibility are easily invaded by sex ratio distorting mutants. Am Nat 160:360–373
Jaenike J (2007) Spontaneous emergence of a new Wolbachia phenotype. Evolution 61:2244–2252
Kamping A, Katju V, Beukeboom LW, Werren JH (2007) Inheritance of gynandromorphism in the parasitic wasp Nasonia vitripennis. Genetics 175:1321–1333
Kremer N, Charif D, Henri H, Bataille M, Prevost G, Kraaijeveld K, Vavre F (2009) A new case of Wolbachia dependence in the genus Asobara: evidence for parthenogenesis induction in Asobara japonica. Heredity 103:248–256
Mitsui H, van Achterberg K, Nordlander G, Kimura MT (2007) Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. J Nat Hist 41:1731–1738
Mouton L, Henri H, Boulétreau M, Profizi N, Vavre F (2004) Virulence, multiple infections and regulation of symbiotic population in the Wolbachia–Asobara tabida symbiosis. Genetics 168:181–189
Mouton L, Henri H, Fleury F (2009) Interactions between coexisting intracellular genomes: mitochondrial density and Wolbachia infection. Appl Environ Microbiol 75:1916–1921
Murata Y, Ideo S, Watada M, Mitsui H, Kimura MT (2009) Genetic and physiological variation among sexual and parthenogenetic populations of Asobara japonica (Hymenoptera: Braconidae), a larval parasitoid of drosophilid flies. Eur J Entomol 106:171–178
R Development Core Team (2009) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Sinkins SP, Braig HR, O’Neill SL (1995) Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc R Soc Lond B 261:325–330
Stouthamer R, Breeuwer JAJ, Hurst GDD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Vavre F, Dedeine F, Quillon M, Fouillet P, Fleury F, Bouletreau M (2001) Within-species diversity of Wolbachia-induced cytoplasmic incompatibility in haplodiploid insects. Evolution 55:1710–1714
Vavre F, Fleury F, Varaldi J, Fouillet P, Bouletreau (2002) Infection polymorphism and cytoplasmic incompatibility in Hymenoptera–Wolbachia associations. Heredity 88:361–365
Zhou W, Rousset F, O’Neill S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B 265:509–515
Acknowledgment
This work was supported by an NWO Veni grant to K.K.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kraaijeveld, K., Reumer, B.M., Mouton, L. et al. Does a parthenogenesis-inducing Wolbachia induce vestigial cytoplasmic incompatibility?. Naturwissenschaften 98, 175–180 (2011). https://doi.org/10.1007/s00114-010-0756-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-010-0756-x