Abstract
Cichlids are an excellent model to study explosive speciation and adaptive radiation. Their evolutionary success has been attributed to their ability to undergo rapid morphological changes related to diet, and their particular breeding biology. Relatively minor changes in morphology allow for exploitation of novel food resources. The importance of phenotypic plasticity and genetically based differences for diversification was long recognized, but their relationship and relative magnitude remained unclear. We compared morphology of individuals of four wild populations of the Lake Tanganyika cichlid Tropheus moorii with their pond-raised F1 offspring. The magnitude of morphological change via phenotypic plasticity between wild and pond-bred F1 fish exceeds pairwise population differences by a factor of 2.4 (mean Mahalanobis distances). The genetic and environmental effects responsible for among population differentiation in the wild could still be recognized in the pond-bred F1 fish. All four pond populations showed the same trends in morphological change, mainly in mouth orientation, size and orientation of fins, and thickness of the caudal peduncle. As between population differentiation was lower in the wild than differentiation between pond-raised versus wild fish, we suggest the narrow ecological niche and intense interspecific competition in rock habitats is responsible for consistent shape similarity, even among long-term isolated populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A central goal in evolutionary biology is answering the question about the origin of phenotypic divergence and the influence of environment on shaping phenotypes (e.g., Via and Lande 1985; Barel 1993; West-Eberhard 2003; 2005). Divergent natural selection has long been put forward as a major mechanism in the evolution of trophic specialization (Barel 1983; Bouton et al. 1997). Thereby natural selection acts on polymorphic traits by sorting variation towards novel niches in conjunction with the origin of novel species. More specifically, the evolution of reproductive isolation between populations by divergent natural selection arising from differences between ecological niches was named ecological speciation. This type of speciation was thought to be a general phenomenon that might occur in allopatry or sympatry, involve many agents of natural selection, and results from a combination of adaptive processes (Schluter 2000, 2001; McKinnon et al. 2004; Rundle and Nosil 2005). Particular circumstances induce periods of intensive innovation, a phenomenon termed adaptive radiation (Sturmbauer 1998; Robinson and Schluter 2000; Schluter 2000). Such periods occurred repeatedly during the history of our planet and are best studied in suitable model systems in which the process is currently ongoing or in an advanced stage. The enormously diverse species flocks of cichlid fishes in the East African Great Lakes are such model systems in which the occurrence and relative importance of parameters promoting speciation can be addressed (Meyer 1993).
Phenotypic variation among individuals and populations may be due to genetic and/or environmental factors. West-Eberhard (1989) defined phenotypic plasticity as follows: the ability of a single genotype to produce an array of alternative phenotypes in response to environmental conditions. This may be manifested in morphology, physiological state, and/or behavior. The study of phenotypic plasticity has progressed significantly over the past few decades (Pigliucci 2005). Plasticity has been hypothesized to act as an important strategy for organisms to cope with environmental variation (Stearns 1989; Scheiner 1993), and a plastic response to a changing environment might be adaptive in that individuals displaying such a response have higher fitness than those that do not (Price et al. 2003). The role of phenotypic plasticity for evolution has been a hotly debated topic since West-Eberhard’s book (2003), in which she postulated that the sequence of developmental plasticity, phenotypic accommodation and subsequently genetic accommodation, is the mechanism responsible for almost all evolutionary novelty, speciation, adaptive radiation, and macroevolution. In other words, evolution would proceed through adaptive developmental phenotypic plasticity. A number of different studies, reviews, and opinions followed, but the exact role of phenotypic plasticity in evolution remains controversial (e.g., De Jong and Crozier 2003; Pigliucci and Murren 2003; Behera and Nanjundiah 2004; de Jong 2005; Chapman et al. 2008).
Phenotypic plasticity in body shape and trophic morphology in response to different food types and feeding orientation was demonstrated for a variety of organisms (reviewed in Via et al. 1995; Agrawal 2001; West-Eberhard 2003). Fishes and especially cichlid fishes have been shown to be particularly plastic in their trophic morphology and behavior (Meyer 1987; Meyer et al. 1990; Wimberger 1991; Day et al. 1994; Huysseune 1995; Robinson and Wilson 1995; Hofmann 2003; Wintzer and Motta 2005; Solem et al. 2006; Aubin-Horth et al. 2007; Burmeister 2007), and this plasticity was put forward as one of the key factors promoting their evolutionary success (Greenwood 1965, 1984; Hoogerhoud 1986; Meyer 1987; Witte et al. 1990; Stauffer and Gray 2004). This group of fishes has successfully colonized several rivers and lakes, and these colonizers split into numerous species by colonizing all thinkable ecological niches. The East African Great lakes are particularly species rich, and each lake comprises hundreds of endemic species. The cichlids of Lake Tanganyika are morphologically, ecologically, and behaviorally the most diverse within the family Cichlidae (Fryer and Iles 1972; Greenwood 1984; Chakrabarty 2005; Koblmüller et al. 2008). To date only few studies on phenotypic plasticity on Lake Tanganyika cichlids exist. These focused on the plasticity of a variety of life history traits (growth, size and time of reproduction, social skills, and cognitive abilities) using the split-brood approach (Taborsky 2006a, b; Arnold and Taborsky 2010; Kotrschal and Taborsky 2010).
The genus Tropheus, of which about 120 color morphs in six nominal species (Poll 1986) are currently described, is an ideal model to study evolutionary processes (Sturmbauer and Meyer 1992; Egger et al. 2007). Tropheus is one of the most abundant algae grazers in the upper littoral zone living in all types of rocky habitats (Sturmbauer et al. 2008). Consequently, sandy or muddy shores and river estuaries are strictly avoided and constitute barriers to gene flow (Sefc et al. 2007). Almost every continuous stretch of rocky shoreline is inhabited by its own color morph. In contrast to coloration, morphology turned out to be highly similar among allopatric populations and sister species (Sturmbauer and Meyer 1992), most probably due to the fact that Tropheus occupies the same niche in all allopatric habitats and due to stabilizing selection. However, recent studies showed that there are small but clear differences between populations in body shape (Maderbacher et al. 2008) as well as in a single viscerocranial element (Postl et al. 2008). Thus, the existence of patterns in the degree of phenotypic plasticity can be addressed by comparing allopatric populations or sister species which are morphologically constrained in similar ways.
This study compared the overall morphology of wild fish and pond-raised F1 offspring of the Lake Tanganyika cichlid Tropheus moorii. To this end, we raised offspring of four populations as independent replicates to test for common effects. This study design tested the hypothesis that environment-induced morphological differences do not affect shape differences among populations (Maderbacher et al. 2008; Postl et al. 2008). We asked if morphological differences among populations remain intact in a standardized pond environment or if morphological plasticity can annihilate population differentiation, in order to explore the relative proportion of genetically fixed and environment-induced effects on body shape. We quantified genetic variation among investigated populations and examined the pattern and extent of plasticity of body shape caused by the pond environment. We considered only a narrow size class of juveniles due to the known sexual dimorphism in adults of the study species T. moorii (Herler et al. 2010).
Materials and methods
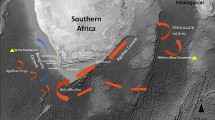
This study is based on specimens of four different populations of T. moorii. In March 2004, population samples were taken at Mbita Island (8°44′S, 31°06′E) and at Nakaku (8°38′S, 30°52′E). Both localities were located in the southern section of the lake. The other two populations were from the central eastern section of the lake. These fish were collected near Ikola (6°41′S, 30°21′E) and Kekese (6°36′S, 30°17′E) in February 2007 (Fig. 1a). The population at Kekese is one of the few in which two Tropheus coexist and the study species T. moorii lives deeper than its ally Tropheus polli. Just after capture, a portion of the catch was sent to the University of Graz alive. Simultaneously, four identical breeding ponds of a size of 2 × 5 m and 70 cm water depth were stocked with wild adults from the four populations next to Lake Tanganyika to produce F1 offspring. For Mbita and Nakaku, each pond was stocked with 25 males and 75 females; for Kekese and Ikola, ponds were stocked with ten males and 30 females. The number of stocked individuals was reduced for the Ikola–Kekese experiment as it turned out that few fish actually bred and thus territorial stress among adults could be reduced. Parental fish were removed after 1 year. Each pond was equipped with a standardized cobble landscape. Fish were fed daily with flake food in addition to naturally available algae growing on rocks within the pond. Ponds were cleaned daily including a water change of 25% of the pond water with freshly pumped lake water (temperature 27°C, pH 9.1). Thus, there was no difference in water chemistry between the wild and the pond environment. F1 offspring were investigated as they reached about the size of our wild-juvenile sample. A small fin clip of each individual was taken for genetic analysis.
Sampling locations and landmark set. a Map of Lake Tanganyika with details on sampling locations and drawings of the four color morphs from Schupke (2004). b Landmark positions for geometric morphometric analysis and the angle (α) describing mouth orientation. 1 Anterior tip of the snout; 2, 3 anterior and posterior insertion of the dorsal fin; 4, 6 upper and lower insertion of caudal fin; 5 midpoint of the origin of the caudal fin; 7, 8 posterior and anterior insertion of the anal fin; 9 insertion of the ventral fin; 10 ventral tip of cleithrum; 11 most ventral point of the border between inter-operculum and sub-operculum; 12 the point where pre-operculum, inter-operculum, and sub-operculum get in contact; 13 upper insertion of the pelvic fin; 14 dorsal origin of the operculum; 15 dorsal end of the pre-opercular groove; 16 and 18 lie at the extreme of the orbit along the antero-posterior body axis; capture the width of the bony orbit. 17 Center of the eye, 19 most posterior point of the lips
For determination of genetic differentiation among investigated populations, we analyzed nine microsatellite loci UNH154, UNH130, Pzep3, UNH908, Pzep2, UME003, UME002, TmoM11, and TmoM27 (Lee et al. 1995; Parker and Kornfield 1996; Zardoya et al. 1996; van Oppen et al. 1997; Carlton et al. 2002; Albertson et al. 2009). Methods of DNA extraction and PCR conditions are described in Koch et al. (2008). The inferred genotypic information (241 Mbita, 177 Nakaku, 201 Kekese, and 207 Ikola) from nine microsatellite loci was evaluated for deviations from Hardy–Weinberg equilibrium and linkage disequilibrium using the software package Arlequin 3.11 (Excoffier et al. 2006). All nine microsatellite loci confirmed Hardy–Weinberg expectations and testing for linkage equilibrium revealed that markers were inherited independently. Genetic characterization was based on estimates of allele and genotype frequencies, gene diversity, and allelic richness. Pairwise FST values were calculated in Arlequin.
For morphological analysis, we restricted our comparisons to a narrow size class between 30 and 65 mm standard length. The data considered in morphometric analysis included 114 juveniles from wild populations (23 Mbita, 22 Nakaku, 32 Kekese, and 19 Ikola) and 335 pond-bred F1 offspring (114 Mbita, 158 Nakaku, 31 Kekese, and 32 Ikola). Digital images of anesthetized specimens were obtained using a flatbed scanner (Herler et al. 2007). Coordinates of 19 landmarks (Fig. 1b) were digitized using TpsDig 2.10 (Rohlf 2006).
We used a geometric morphometric approach based on Procrustes methods (Bookstein 1996; Dryden and Mardia 1998). By using residual components of regression of shape on size, the allometric component of within-group variation as well as size-related differences between groups were considered. To examine the variation among populations and environment, we performed a canonical variate analysis (CVA; Mardia et al. 1979). An unrooted neighbor-joining tree based on Mahalanobis distances was created using the program PAST (Hammer et al. 2001). A principal component analysis (PCA) based on the covariance matrix of landmark data, including all individuals, was carried out to investigate overall effects of environment on morphology. A discriminant function analysis was done to find features of shape that have a maximal difference between environments relative to the variation within populations. Analyses were carried out in MorphoJ (Klingenberg 2011). In addition to the comparisons of overall shape, we applied discrete measurements in form of interlandmark distances (ILD). All possible distances between 19 landmarks (Modicos; Carvajal-Rodríguez and Rodríguez 2005) were generated. Selected distances (distance between landmarks 2 and 3; 4 and 6; 7 and 8) were related to standard length and compared by Kruskal–Wallis tests among wild juveniles and pond-bred F1 generation. To test if there are any differences in mouth orientation, we additionally measured the angle α between landmarks 1, 2, and 19 for a random sub-sample of 40 wild juveniles and 40 pond F1 offspring on digital images in TpsDig 2.10. Statistical analyses were performed in PAST (Hammer et al. 2001).
Results
Moderate genetic differentiation was observed between Mbita and Nakaku (F ST = 0.055; p < 0.0001), and Ikola and Kekese (F ST = 0.049, p < 0.0001), while high genetic differentiation was detected among eastern versus southern populations (F ST > 0.25, p < 0.0001; Fig. 2b, Table S1). Morphometric data showed a linear size–shape relationship (Fig. 2a), albeit we restricted our comparisons to a narrow size class between 30 and 65 mm standard length.
Genetic and morphological discrimination between populations and environments. a Regression of shape (Procrustes coordinates) on centroid size (estimating the specimen size) including wild male, female, and juvenile individuals of the four study populations. b Unrooted tree by the neighbor-joining method on the F ST distance matrix based upon nine microsatellite loci of the populations at Nakaku, Mbita, Kekese, and Ikola. c Unrooted neighbor-joining tree based on Mahalanobis distances obtained from a CVA on landmark data of the four populations in two different environments. d Deformation grids according to the two CV axes
Overall, the pattern of morphological differentiation among populations paralleled that seen with genetic differentiation (Fig. 2c). There is also high morphological differentiation between southern and eastern populations. However, analysis of pond-bred juveniles of the four study populations yielded a surprisingly large change in morphology reflected by 2.4-fold larger Mahalanobis distances among wild versus pond-bred specimens, irrespective of population (Fig. 2c; Table S2). The deformation grids shown in Fig. 2d highlight these marked differences, whereby canonical variate axis 1 predominantly reflects the differences between the two environments and CV axis 2 those between populations. When the two population pairs from the eastern and southern region were analyzed separately, the CVA scatter plots visualized the notable excess of environmentally induced plasticity in relation to the morphological divergence among the wild populations (Fig. 3a, b). In both CVA scatter plots, wild juveniles and pond-bred F1 offspring could be clearly separated along CV axis 1, which means the pond effect goes in consistent direction in all four populations. Populations are clearly separated from each other along CV axis 2, except the wild Ikola and Kekese individuals.
Shape change as a cause of different environment. a Canonical variate analysis on shape data including wild juveniles and pond-bred F1 offspring of Kekese and Ikola (Kekese wild juveniles shaded circles; Ikola wild juveniles open circles; Kekese pond offspring shaded squares; Ikola pond offspring open squares) and b Mbita and Nakaku (Mbita wild juveniles shaded circles; Nakaku wild juveniles open circles; Mbita pond offspring shaded squares; Nakaku pond offspring open squares). c Principal component analysis on shape data including all wild juveniles (filled circles) and pond-bred F1 offspring (shaded squares) of four populations of T. moorii. d Demonstration of shape changes in different environments using a warped outline drawing (scaling factor = 0.08) according to PC 1
To test for the generality of the phenomenon of a pond effect, a PCA of the complete landmark data set was carried out without pre-defining group assignment.
Wild juveniles and pond-bred F1 offspring overlapped somewhat along both of the first two PC factors, but a relatively strong differentiation was nevertheless observed in multivariate space with most wild individuals positioned to the lower left (more negative) of the graph, and most pond-bred F1 individuals to the upper right (more positive) (Fig. 3c). We found that the most pronounced shape changes concerned the orientation of the mouth, a backward movement of the origin of the dorsal fin and the anal fin resulting in a surface reduction and a narrower caudal peduncle (Fig. 3d). In all four populations, the discriminant function could separate wild and pond juveniles without any overlap, so that every specimen can be correctly allocated. Individually, each population showed the same direction and nearly the same magnitude of shape change from wild to pond individuals (Fig. 4).
Selected body measurements were significantly different (p < 0.0001) between wild juveniles and pond-bred F1 offspring and could be equalized with traditional measurements (ILD 2–3 = dorsal fin base length, 4–6 = caudal peduncle height, and 7–8 = anal fin base length; Fig. 5a–c). The angle between insertion of the dorsal fin, tip of the snout, and most posterior tip of the lips was about 4° larger in pond-bred F1 juveniles than in wild juveniles, meaning that wild fish had a more inferior mouth position (Fig. 5d).
Comparison of discrete measurements between the two environments (pond offspring open squares; wild juveniles shaded squares. Box–whisker plots of three significantly different interlandmark distances (p < 10−41): a dorsal fin base length, b caudal peduncle height, and c anal fin base length (wild juveniles n = 96, pond offspring n = 335) and of measurements of d the angle α between landmarks 2, 1, and 19 (wild juveniles n = 40, pond offspring n = 40) on specimens from the two environments (p = 3.5 × 10−6)
Discussion
The innovative aspect of our study was to consider the degree and pattern of phenotypic plasticity in relation to morphological differences found in natural populations which are subject to similar selective forces. Therefore, we contrasted the wave-exposed and competitor- and predator-driven natural rock environment of Tropheus with the fully calm and predator-free environment of a concrete pond with standardized rock architecture and feeding regime. In this environment, we produced F1 offspring of four different populations and color morphs, which were tested for Hardy–Weinberg equilibrium. This design was chosen to demonstrate the relative magnitude of phenotypic and genetic contribution to population differences in evolving populations. As argued by Price et al. (2003), phenotypic plasticity may be a fitness-relevant trait subject to natural selection. In fact, the degree of morphological plasticity can have profound influences on evolutionary and ecological outcomes. It could, for example, influence the ability to track environmental changes and thus regulate the potential for character divergence. Furthermore, competitive interactions between species might be altered if one of the competitors has a highly plastic morphology that enables it to efficiently utilize a wider range of resources than would otherwise be possible (Olsson and Eklöv 2005). It was also shown that phenotypic plasticity involves life history traits with adaptive significance such as growth, size and time of reproduction, social skills, and cognitive abilities (Taborsky 2006a, b; Arnold and Taborsky 2010; Kotrschal and Taborsky 2010).
Our study species is a highly specialized algae feeder that lives in a variety of rock habitats, ranging from moderately sloping cobble shores to steeply descending solid rock, so that phenotypic plasticity might be relevant for its fitness and evolution. In previous studies, we have shown that populations of Tropheus could be discriminated based on subtle morphological differences. Even if such low levels of variation are expected between populations at early stages of evolutionary divergence, they may even be superimposed by phenotypic plasticity. Thus, the genetic component of population variation is particularly difficult to demonstrate in evolutionarily young populations. Therefore, it is crucial to assess the degree of phenotypic plasticity in a standardized design. Our experiment indeed showed that the magnitude of morphological change via phenotypic plasticity can exceed population differences by a factor of 2.4 even though populations of Tropheus were separated for about 100,000 years (Sturmbauer et al. 2005). At the same time, we demonstrated that there are also population-specific differences that remain intact in individuals raised in a standardized environment.
The observed changes in the common pond environment were consistent in all four study populations. The most impressive change concerns orientation of the mouth. In cichlids, but also in other fish families, structural differences in trophic morphology have been related more to the way food is captured and processed than to the type of food consumed (Barel 1983; Yamaoka 1997; Wintzer and Motta 2005). Hence, the change in the angle of the mouth could be interpreted as a response to less scraping from the rocks, in addition to more sucking in the water column, as a consequence of the availability of flake food in the ponds. In the wild, an inferior subterminal mouth is characteristic for Tropheus and is used to scrape algae from rocks. Various studies demonstrate linkage between functional morphology in cichlid jaws and differences in feeding performance for Lake Victoria cichlids (Bouton et al. 1997, 1998, 2002). Albertson and Kocher (2001) compared jaw morphologies between two closely related cichlid species of Lake Malawi and found that many aspects of shape differences clearly reflect different modes of feeding. Fin size and shape is expected to affect swimming performance. High aspect ratios, defined as the square of the span divided by the fin area, characterize fast-swimming fishes, while low ratios are measured in fishes with low swimming performance but better maneuvering abilities (Weihs 1989; Videler 1993). Changes in fin structure of pond individuals could be a response to more quiet water and lack of predation within the ponds. Another interesting aspect concerns the population of Kekese, as it lives in deeper water than the remaining study populations due to the sympatry of a second Tropheus. While the pond effect goes in consistent direction (Fig. 3a, left side; Fig. 4), there are population-specific components in body morphology. We observed a somewhat distinct segregation among the populations from Ikola and Kekese (Fig. 3a, right side). This may be due to the fact that the Kekese population shifted towards greater water depths due to the presence of a second Tropheus species in its habitat, but this remains to be tested in a future study.
From the non-random pattern of the observed morphological changes, we conclude that they were not caused by a simple release of selection pressure but rather as a plastic response to a novel environment. A second issue concerns the magnitude of change between the wild populations and the pond fish. We observed that the magnitude of morphological change via phenotypic plasticity outreaches that found among natural populations by a factor of 2.4. This indicates that Tropheus has a higher potential for phenotypic plasticity than the degree of population variation observed. Our interpretation concerning the evolutionary significance of phenotypic plasticity is that there is intense selection within each of those spatially and genetically isolated species communities, keeping Tropheus in its relatively narrow trophic niche. This type of stabilizing selection is enforced by both biological interactions with other species in the habitat and similar selective forces from abiotic characteristics of the rock habitats of the four study populations. Because observed morphological variation among the four study populations remained intact after being exposed to a common environment, we also conclude that part of the morphological differences among Tropheus populations are indeed genetically determined.
To conclude, our study connects to West-Eberhard’s hypothesis that the sequence of developmental plasticity, phenotypic accommodation, and subsequently genetic accommodation might be the most important mechanism for the origin of evolutionary novelty, speciation, adaptive radiation, and macroevolution. In allopatric populations of Tropheus, the scope of phenotypic plasticity is not fully exploited, so that there is still potential for adaptive developmental phenotypic plasticity should the environment change more radically. An organism’s phenotype is affected by both internal (genetic and developmental processes) and environmental factors (Albertson and Kocher 2006) and thus both can contribute to the fitness of an individual. Ontogenetic plasticity can be subject to natural selection, as each genotype has the potential to produce a range of phenotypes, as a second-order response to more short-term fluctuations of the environment. However, current research in adaptation is predominantly focused on uncovering the genetic basis of specific traits of evolutionary importance through quantitative genetic analysis. A more explicit consideration of phenotypic plasticity will necessarily complicate such approaches, and we suggest that it is equally important to assess the scope of phenotypic plasticity when addressing the genetic basis of differentiation.
References
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Albertson RC, Kocher TD (2001) Assessing morphological differences of an adaptive trait: a landmark-based morphometric approach. J Exp Zool 289:385–403
Albertson RC, Kocher TD (2006) Genetic and developmental basis of cichlid trophic diversity. Heredity 97:211–221
Albertson RC, Streelman JT, Kocher TD (2009) Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc Natl Acad Sci 100:5252–5257
Arnold C, Taborsky B (2010) Social experience in early ontogeny has lasting effects on social skills in cooperatively breeding cichlids. Anim Behav 79:621–630
Aubin-Horth N, Desjardins JK, Martei YM, Balshine S, Hofmann HA (2007) Masculinized dominant females in a cooperatively breeding species. Mol Ecol 16:1349–1358
Barel CDN (1983) Towards a constructional morphology of cichlid fishes (Teleostei, Perciformes). Neth J Zool 33:357–424
Barel CDN (1993) Concepts of an architectonic approach to transformation morphology. Acta Biotheorethica 41:345–381
Behera N, Nanjundiah V (2004) Phenotypic plasticity can potentiate rapid evolutionary change. J Theor Biol 226:177–184
Bookstein FL (1996) Applying landmark methods to biological outline data. In: Mardia KV, Gill CA, Dryden IL (eds) Image fusion and shape variability. University of Leeds Press, Leeds, pp 79–87
Bouton N, Seehausen O, van Alphen JJM (1997) Resource partitioning among rock-dwelling haplochromines (Pisces: Cichlidae) from Lake Victoria. Ecol Freshw Fish 6:225–240
Bouton N, van Os N, Witte F (1998) Feeding performance of Lake Victoria rock cichlids: testing predictions from morphology. J Fish Biol 53(suppl A):118–127
Bouton N, Witte F, van Alphen JJM (2002) Experimental evidence for adaptive phenotypic plasticity in a rock-dwelling cichlid fish from Lake Victoria. Biol J Linn Soc 77:185–192
Burmeister SS (2007) Genomic responses to behavioral interactions in an African cichlid fish: mechanisms and evolutionary implications. Brain Behav Evol 70:247–256
Carlton KL, Streelman JT, Lee BY, Garnhart N, Kidd M, Kocher TD (2002) Rapid isolation of CA microsatellites from the tilapia genome. Genetics 33:140–144
Carvajal-Rodríguez A, Rodríguez MG (2005) Morphometric and distance computation software oriented for evolutionary studies. Online J Bioinform 6:34–41
Chakrabarty P (2005) Testing conjectures about morphological diversity in Cichlids of Lake Malawi and Tanganyika. Copeia 2:359–373
Chapman L, Albert J, Galis F (2008) Developmental plasticity, genetic differentiation, and hypoxia-induced trade-offs in an African cichlid fish. Open Evol J 2:75–88
Day T, Pritchard J, Schluter D (1994) Ecology and genetics of phenotypic plasticity: a comparison of two sticklebacks. Evolution 48:1723–1734
De Jong G (2005) Evolution of phenotypic plasticity, patterns of plasticity and the emergence of ecotypes. New Phytol 166:101–118
De Jong G, Crozier RH (2003) A flexible theory of evolution. Nature 424:16–17
Dryden IL, Mardia KV (1998) Statistical shape analysis. Wiley, New York
Egger B, Koblmüller S, Sturmbauer C, Sefc KM (2007) Nuclear and mitochondrial data reveal different evolutionary processes in the Lake Tanganyika cichlid genus Tropheus. BMC Evol Biol 7:137
Excoffier L, Laval G, Schneider S (2006) Arlequin ver 3.11: an integrated software package for population genetics data analysis. Computational and Molecular Population Genetics Lab (CMPG). Institute of Zoology University of Berne, Switzerland
Fryer G, Iles TD (1972) The cichlid fishes of the Great Lakes of Africa. Their biology and evolution. Oliver & Boyd, Edinburgh
Greenwood PH (1965) Environmental effects on the pharyngeal mill of a cichlid fish, Astatoreochromis alluaudi and their taxonomic implications. Proc Biol J Linn Soc 176:1–10
Greenwood PH (1984) African cichlids and evolutionary theories. In: Echelle AA, Kornfield I (eds) Evolution of fish species flocks. University of Maine Press, Orono, pp 141–154
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeont Electr 4(1):9
Herler J, Lipej L, Makovec T (2007) A simple technique for digital imaging of live and preserved small fish specimens. Cybium 31:39–44
Herler J, Kerschbaumer M, Mitteroecker P, Postl L, Sturmbauer C (2010) Sexual dimorphism and population divergence in the Lake Tanganyika cichlid fish genus Tropheus. Front Zool 7(1):4
Hofmann HA (2003) Functional genomics of neural and behavioral plasticity. J Neurobiol 54:272–282
Hoogerhoud RJC (1986) Taxonomic and ecological aspects of morphological plasticity of molluscivorous haplochromines (Pisces, Cichlidae). Ann Mus Roy Aft Centr Sc Zool 251:131–134
Huysseune A (1995) Phenotypic plasticity in the lower pharyngeal jaw dentition of Astatoreochromis alluaudi (Teleostei: Cichlidae). Arch Oral Biol 40:1005–1014
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources. doi:10.1111/j.1755-0998.2010.02924.x
Koblmüller S, Sefc KM, Sturmbauer C (2008) The Lake Tanganyika cichlid species assemblage: recent advantages in molecular phylogenetics. Hydrobiologia 615:5–20
Koch M, Hadfield JJ, Sefc KM, Sturmbauer C (2008) Pedigree reconstruction in wild cichlid fish populations. Mol Ecol 17:4500–4511
Kotrschal A, Taborsky B (2010) Environmental change enhances cognitive abilities in fish. PLOS Biol 8: doi:10.1371/journal.pbio.1000351
Lee WJ, Conroy J, Huntting Howell W, Kocher TD (1995) Structure and evolution of teleost mitochondrial control regions. J Mol Evol 41:54–66
Maderbacher M, Bauer C, Herler J, Postl L, Makasa L, Sturmbauer C (2008) Assessment of traditional versus geometric morphometrics for discriminating populations of the Tropheus moorii species complex (Teleostei: Cichlidae), a Lake Tanganyika model for allopatric speciation. J Zool Syst Evol Res 46:153–161
Mardia KV, Kent JT, Bibby JM (1979) Multivariate analysis. Academic, London
McKinnon J, Mori S, Blackman BK, David L, Kingsley DM, Jamieson L, Chou J, Schluter D (2004) Evidence for ecology’s role in speciation. Nature 429:294–298
Meyer A (1987) Phenotypic plasticity and heterochrony in Cichlasoma managuense (Pisces, Cichlidae) and their implications for speciation in cichlid fishes. Evolution 41:1357–1369
Meyer A (1993) Phylogenetic relationships and evolutionary processes in East African cichlid fishes. TREE 8:279–284
Meyer A, Kocher TD, Basasibwaki P, Wilson AC (1990) Monophyletic origin of Lake Victoria cichlid fishes suggested by mitochondrial DNA sequences. Nature 347:550–553
Olsson J, Eklöv P (2005) Habitat structure, feeding mode and morphological reversibility: factors influencing phenotypic plasticity in perch. Evol Ecol Res 7:1109–1123
Parker A, Kornfield I (1996) Polygynandry in Pseudotropheus zebra, a cichlid fish from Lake Malawi. Env Biol Fish 47:345–352
Pigliucci M (2005) Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol 20:481–486
Pigliucci M, Murren CJ (2003) Genetic assimilation and a possible evolutionary paradox, can macroevolution sometimes be so fast as to pass us by? Evolution 57:1455–1464
Poll M (1986) Classification des Cichlidae du lac Tanganika: tribus, genres et espéces. In: XLV T (ed) Fascicule, 2nd edn. Académie Royale de Belgique, Brussels
Postl L, Herler J, Bauer C, Maderbacher M, Makasa L, Sturmbauer C (2008) Geometric morphometrics applied to viscerocranial bones in three populations of the Lake Tanganyika cichlid fish Tropheus moorii. J Zool Syst Evol Res 46:240–248
Price TD, Qvarnstroem A, Irwin DE (2003) The role of phenotypic plasticity in driving genetic evolution. Proc R Soc Lond B 270:1433–1440
Robinson BW, Schluter D (2000) Natural selection and the evolution of adaptive genetic variation in northern freshwater fishes. In: Mousseau T, Sinvero B, Endler JA (eds) Adaptive genetic variation in the wild. Oxford University Press, Oxford, pp 65–94
Robinson BW, Wilson DS (1995) Experimentally induced morphological diversity in Trinidadian guppies (Poecilia reticulata). Copeia 2:294–305
Rohlf FJ (2006) TPS software series. Department of Ecology and Evolution, State University of New York, Stony Brook
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352
Scheiner SM (1993) Genetics and evolution of phenotypic plasticity. Ann Rev Ecolog Syst 24:35–68
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, New York
Schluter D (2001) Ecology and the origin of species. Trends Ecol Evol 16:372–380
Sefc KM, Baric S, Salzburger W, Sturmbauer C (2007) Species-specific population structure in rock-specialized sympatric cichlid species in Lake Tanganyika, East Africa. J Mol Evol 64:33–49
Solem O, Berg OK, Kjosnes AJ (2006) Inter- and intra-population morphological differences between wild and farmed Atlantic salmon juveniles. J Fish Biol 69:1466–1481
Stauffer JR, Gray EVS (2004) Phenotypic plasticity: its role in trophic radiation and explosive speciation in cichlids (Teleostei: Cichlidae). Anim Biol 54:137–158
Stearns S (1989) The evolutionary significance of phenotypic plasticity. Bioscience 39:436–445
Sturmbauer C (1998) Explosive speciation in cichlid fishes of the African Great Lakes: a dynamic model of adaptive radiation. J Fish Biol 53(Supplement A):18–36
Sturmbauer C, Meyer A (1992) Genetic divergence, speciation and morphological stasis in a lineage of African cichlid fishes. Nature 358:578–581
Sturmbauer C, Koblmüller S, Sefc KM, Duftner N (2005) Phylogeographic history of the genus Tropheus, a lineage of rock-dwelling cichlid fishes endemic to Lake Tanganyika. Hydrobiologia 542:335–366
Sturmbauer C, Fuchs C, Harb G, Damm E, Duftner N, Maderbacher M, Koblmüller S (2008) Abundance, distribution and territory areas of rock-dwelling Lake Tanganyika cichlid species. Hydrobiologia 615:57–68
Taborsky B (2006a) The influence of juvenile an adult environments on life history trajectories. Proc R Soc B 273:741–750
Taborsky B (2006b) Mothers determine offspring size in response to own juvenile growth conditions. Biol Lett 2:225–228
Van Oppen MJH, Rico C, Deutsch JC, Turner GF, Hewitt GM (1997) Isolation and characterization of microsatellite loci in the cichlid fish Pseudotropheus zebra. Mol Ecol 6:387–388
Via S, Lande R (1985) Genotype–environment interaction and the evolution of phenotypic plasticity. Evolution 39(3):505–522
Via S, Gomulkiewicz R, DeJong G, Scheiner SM, Schlichting CD, VanTiendern PH (1995) Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol Evol 10:212–217
Videler JJ (1993) Fish swimming. Chapman and Hall, London
Weihs D (1989) Design features and mechanics of axial locomotion in fish. Amer Zool 29(1):151–160
West-Eberhard MJ (1989) Phenotypic plasticity and the origins of diversity. Ann Rev Ecolog Syst 20:249–278
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, New York
West-Eberhard MJ (2005) Developmental plasticity and the origin of species differences. Proc Natl Acad Sci 102:6543–6549
Wimberger PH (1991) Plasticity of jaw and skull morphology in the neotropical cichlids Geophagus brasiliensis and G. steindachneri. Evolution 45:1545–1563
Wintzer AP, Motta PJ (2005) Diet-induced phenotypic plasticity in the skull morphology of hatchery-reared Florida largemouth bass, Micropterus salmoides floridanus. Ecol Freshw Fish 14:311–318
Witte F, Barel CDN, Hoogerhoud RJC (1990) Phenotypic plasticity of anatomical structures and its ecomorphological significance. Neth J Zool 40:278–298
Yamaoka K (1997) Trophic ecomorphology of Tanganyikan cichlids. In: Kawanabe H, Hori M, Nagoshi M (eds) Fish communities in Lake Tanganyika. Kyoto University Press, Kyoto, pp 25–56
Zardoya R, Vollmer DM, Craddock C, Streelman JT, Karl S, Meyer A (1996) Evolutionary conservation of microsatellite flanking regions and their use in resolving the phylogeny of cichlid fishes (Pisces: Perciformes). Proc Roy Soc B 263:1589–1598
Acknowledgments
We thank Stephan Koblmüller and Steven Weiss for suggestions and critical readings of the manuscript. K.M., P.L., and C.S. were supported by the Austrian Science Foundation (Grant P20994-B03) as well as from the Commission for Interdisciplinary Ecological Studies of the Austrian Academy of Sciences (Project 2007-04).
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kerschbaumer, M., Postl, L., Koch, M. et al. Morphological distinctness despite large-scale phenotypic plasticity—analysis of wild and pond-bred juveniles of allopatric populations of Tropheus moorii . Naturwissenschaften 98, 125–134 (2011). https://doi.org/10.1007/s00114-010-0751-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-010-0751-2