Abstract
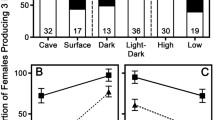
The majority of studies on ecological speciation in animals have investigated the divergence caused by biotic factors like divergent food sources or predatory regimes. Here, we examined a system where ecological speciation can clearly be ascribed to abiotic environmental gradients of naturally occurring toxic hydrogen sulfide (H2S). In southern Mexico, two genera of livebearing fishes (Poeciliidae: Poecilia and Gambusia) thrive in various watercourses with different concentrations of H2S. Previous studies have revealed pronounced genetic differentiation between different locally adapted populations in one species (Poecilia mexicana), pointing towards incipient speciation. In the present study, we examined female reproductive life-history traits in two species pairs: Gambusia sexradiata (from a nonsulfidic and a sulfidic habitat) and Gambusia eurystoma (sulfide-endemic), as well as P. mexicana (nonsulfidic and sulfidic) and Poecilia sulphuraria (sulfide endemic). We found convergent divergence of life-history traits in response to sulfide; most prominently, extremophile poeciliids exhibit drastically increased offspring size coupled with reduced fecundity. Furthermore, within each genus, this trend increased with increasing sulfide concentrations and was most pronounced in the two endemic sulfur-adapted species. We discuss the adaptive significance of large offspring size in toxic environments and propose that divergent life-history evolution may promote further ecological divergence through isolation by adaptation.



Similar content being viewed by others
References
Alvarez del Villar J (1948) Descripción de una nueva especie de Mollienisia capturada en Baños del Azufre, Tabasco (Pisces, Poeciliidae). An Esc Nac Cienc Biol 5:275–281
Anderson J, Skorping A, Stork NE (1990) Sympatric speciation by habitat specialization and parasitism in carabid beetles. In: Stork NE (ed) The role of ground beetles in ecological and environmental studies. Intercept, Andover, pp 21–29
Antonovics J (2006) Evolution in closely adjacent plant populations X: long-term persistence of prereproductive isolation at a mine boundary. Heredity 97:33–37
Bagarinao T (1992) Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquat Toxicol 24:21–62
Bashey F (2006) Cross-generational environmental effects and the evolution of offspring size in the Trinidadian guppy Poecilia reticulata. Evolution 60:348–361
Bashey F (2008) Competition as a selective mechanism for larger offspring size in guppies. Oikos 117:104–113
Brockelman WY (1975) Competition, the fitness of offspring, and optimal clutch size. Am Nat 109:677–699
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Dzikowski R, Hulata G, Harpaz S, Karplu I (2004) Inducible reproductive plasticity of the guppy Poecilia reticulata in response to predation cues. J Exp Zool 301A:776–782
Endler JA (1995) Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol Evol 10:22–29
Fontanier ME, Tobler M (2009) A morphological gradient revisited: cave mollies vary not only in eye size. Environ Biol Fish (in press)
Fuller RC, McGhee KE, Schrader M (2007) Speciation in killifish and the role of salt tolerance. J Evol Biol 20:1962–1975
Gosline AK, Rodd FH (2008) Predator-induced plasticity in guppy (Poecilia reticulata) life history traits. Aquat Ecol 42:693–699
Grieshaber MK, Völkel S (1998) Animal adaptations for tolerance and exploitation of poisonous sulfide. Ann Rev Physiol 60:33–53
Hatfield T, Schluter D (1999) Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution 53:866–873
Haynes JL (1995) Standardized classification of poeciliid development for life-history studies. Copeia 1995:147–154
Hays C (2007) Adaptive phenotypic differentiation across the interstitial gradient in the alga Silvetia compressa. Ecology 88:149–157
Hendry AP, Nosil P, Rieseberg LH (2007) The speed of ecological speciation. Funct Ecol 21:455–464
Heulett ST, Weeks SC, Meffe GK (1995) Lipid dynamics and growth relative to resource level in juvenile eastern mosquitofish (Gambusia holbrooki: Poeciliidae). Copeia 1995:97–104
Hoffmann AA, Parsons PA (1997) Extreme environmental change and evolution. Cambridge University Press, Cambridge
Hubbs C (1991) Intrageneric “cannibalism” in Gambusia. Southwest Nat 36:153–157
Hubbs C (1992) Geographic variation in cannibalism of congeneric young by Gambusia adults. Proc Desert Fishes Counc 22:43–52
Hubbs C (1995) Geographic variation in life history traits of Gambusia species. Proc Desert Fishes Counc 27:1–21
Jiménez-Ambriz G, Petit C, Bourrié I, Dubois S, Olivieri I, Ronce O (2007) Life history variation in the heavy metal tolerant plant Thlaspi caerulescens growing in a network of contaminated and noncontaminated sites in southern France: role of gene flow, selection and phenotypic plasticity. New Phytol 173:199–215
Langerhans RB, Gifford ME, Joseph EO (2007) Ecological speciation in Gambusia fishes. Evolution 61:2056–2074
Lexer C, Fay MF (2005) Adaptation to environmental stress: a rare or frequent driver of speciation? J Evol Biol 18:893–900
Lydeard C, Wooten MC, Meyer A (1995) Cytochrome b sequence variation and a molecular phylogeny of the livebearing fish genus Gambusia (Cyprinodontiformes: Poeciliidae). Can J Zool 73:213–227
MacNair MR, Christie P (1983) Reproductive isolation as a pleiotropic effect of copper tolerance in Mimulus guttatus. Heredity 50:295–302
Meffe GK, Snelson FF Jr (1989) An ecological overview of poeciliid fishes. In: Meffe GK, Snelson FF Jr (eds) Ecology & evolution of livebearing fishes (Poeciliidae). Prentice Hall, Englewood Cliffs, pp 13–31
Miller RR (1975) Five new species of Mexican poeciliid fishes of the genera Poecilia, Gambusia, and Poeciliopsis. Occ Pap Mus Zool Univ Michigan 672:1–44
Nesbit DH, Meffe GK (1993) Cannibalism frequencies in wild populations of the Eastern mosquitofish (Gambusia holbrooki, Poeciliidae) in South Carolina. Copeia 1993:867–870
Nosil P, Crespi BJ (2006) Experimental evidence that predation promotes divergence in adaptive radiation. Proc Natl Acad Sci USA 103:9090–9095
Nosil P, Funk DJ, Ortiz-Barrientos D (2009a) Divergent selection and heterogeneous genomic divergence. Mol Ecol 18:375–402
Nosil P, Harmon LJ, Seehausen O (2009b) Ecological explanations for (incomplete) speciation. Trends Ecol Evol 24:145–156
Nyman T, Bokma F, Kopelke JP (2007) Reciprocal diversification in a complex plant–herbivore–parasitoid food web. BMC Biol 5:49
Parker GA, Begon M (1986) Optimal egg size and clutch size: effects of environment and maternal phenotype. Am Nat 128:573–592
Plath M, Tobler M (2009) The evolutionary ecology of the cave molly (Poecilia mexicana, Poeciliidae) from the Cueva del Azufre system. In: Trajano E, Bichuette ME, Kapoor BG (eds) The biology of subterranean fishes. Science, Enfield in press
Plath M, Parzefall J, Schlupp I (2003) The role of sexual harassment in cave- and surface-dwelling populations of the Atlantic molly, Poecilia mexicana (Poeciliidae, Teleostei). Behav Ecol Sociobiol 54:303–309
Plath M, Parzefall J, Körner KE, Schlupp I (2004) Sexual selection in darkness? Female mating preferences in surface- and cave-dwelling Atlantic mollies, Poecilia mexicana (Poeciliidae, Teleostei). Behav Ecol Sociobiol 55:596–601
Plath M, Hauswaldt S, Moll K, Tobler M, García de León FJ, Schlupp I, Tiedemann R (2007a) Local adaptation and pronounced genetic differentiation in an extremophile fish, Poecilia mexicana, inhabiting a Mexican cave with toxic hydrogen sulfide. Mol Ecol 16:967–976
Plath M, Tobler M, Riesch R, García de León FJ, Giere O, Schlupp I (2007b) Survival in an extreme habitat: the role of behaviour and energy limitation. Naturwissenschaften 94:991–996
Ptacek MB, Breden F (1998) Phylogenetic relationships among the mollies (Poeciliidae: Poecilia: Mollienesia group) based on mitochondrial DNA sequences. J Fish Biol 53:64–81
Rajakaruna N, Baldwin BG, Chan R, Desrochers AM, Bohm BA, Whitton J (2003) Edaphic races and phylogenetic taxa in the Lasthenia californica complex (Asteraceae: Heliantheae): an hypothesis of parallel evolution. Mol Ecol 12:1675–1679
Reznick DN, Endler JA (1982) The impact of predation on life-history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36:160–177
Reznick DN, Miles DB (1989) A review of life history patterns in poeciliid fishes. In: Meffe GK, Snelson FF Jr (eds) Ecology & evolution of livebearing fishes (Poeciliidae). Prentice Hall, Englewood Cliffs, pp 125–148
Reznick DN, Bryant MJ, Bashey F (2002) r- and K-selection revisited: the role of density, resources, and environmental fluctuations in life-history evolution. Ecology 83:1509–1520
Riesch R, Schlupp I, Tobler M, García de León FJ, Plath M (2006) Reduction of the association preference for conspecifics in cave-dwelling Atlantic mollies, Poecilia mexicana. Behav Ecol Sociobiol 60:794–802
Riesch R, Duwe V, Herrmann N, Padur L, Ramm A, Scharnweber K, Schulte M, Schulz-Mirbach T, Ziege M, Plath M (2009a) Variation along the shy–bold continuum in extremophile fishes (Poecilia mexicana, P. sulphuraria). Behav Ecol Sociobiol 63:1515–1526
Riesch R, Tobler M, Plath M, Schlupp I (2009b) Offspring number in a livebearing fish (Poecilia mexicana, Poeciliidae): reduced fecundity and reduced plasticity in a population of cave mollies. Environ Biol Fish 84:89–94
Riesch R, Plath M, Schlupp I (2009c) Toxic hydrogen sulfide and dark caves: life-history adaptations to extreme environments in a livebearing fish (Poecilia mexicana, Poeciliidae). Ecology (in press)
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255
Rosales-Lagarde L, Campbell A, Boston PJ, Stafford KW (2008) Sulfur and oxygen isotopes: evidence of H2S spring sources, southern Mexico. Geochim Cosmochim Acta 72:A805
Rundle HD (2002) A test of ecologically dependent postmating isolation between sympatric sticklebacks. Evolution 56:322–329
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, Oxford
Schluter D (2001) Ecology and the origin of species. Trends Ecol Evol 16:372–380
Sibly R, Calow P (1983) An integrated approach to life-cycle evolution using selective landscapes. J Theoret Biol 102:527–547
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Tobler M (2008) Divergence in trophic ecology characterizes colonization of extreme habitats. Biol J Linn Soc 95:517–528
Tobler M, Plath M (2009) Living in extreme environments. In: Evans J, Pilastro A, Schlupp I (eds) Ecology and evolution of poeciliid fishes. Chicago University Press, Chicago in press
Tobler M, Schlupp I, Heubel KU, Riesch R, García de León FJ, Giere O, Plath M (2006) Life on the edge: hydrogen sulfide and the fish communities of a Mexican cave and surrounding waters. Extremophiles 10:577–585
Tobler M, DeWitt TJ, Schlupp I, García de León FJ, Herrmann R, Feulner PGD, Tiedemann R, Plath M (2008a) Toxic hydrogen sulfide and dark caves: phenotypic and genetic divergence across two abiotic environmental gradients in Poecilia mexicana. Evolution 62:2643–2659
Tobler M, Riesch R, García de León FJ, Schlupp I, Plath M (2008b) Two endemic and endangered fishes, Poecilia sulphuraria (Alvarez, 1948) and Gambusia eurystoma Miller, 1975 (Poeciliidae, Teleostei) as only survivors in a small sulphidic habitat. J Fish Biol 72:523–533
Tobler M, Riesch R, Tobler CM, Schulz-Mirbach T, Plath M (2009a) Natural and sexual selection against immigrants maintains differentiation among micro-allopatric populations. J Evol Biol (in press)
Tobler M, Riesch R, Tobler CM, Plath M (2009b) Compensatory behavior in response to sulphide-induced hypoxia affects time budgets, feeding efficiency, and predation risk. Evol Ecol Res 11:935–948
Weeks SC, Gaggiotti OE (1993) Patterns of offspring size at birth in clonal and sexual strains of Poeciliopsis (Poeciliidae). Copeia 1993:1003–1009
Acknowledgements
We thank M. and C.M. Tobler for their help in the field. L.D. Devenport, J.F. Kelly, R.B. Langerhans, E. Marsh-Matthews, L.J. Weider, and an anonymous reviewer helped to improve previous drafts of this manuscript with their valuable comments. R. Riesch thanks D. Reznick for his instruction on life-history sampling techniques. The Mexican Government (Permiso de Pesca de Fomento: DGOPA/16986/191205/8101, DGOPA/02232/230706/1079, DGOPA.06192.240608.-1562, and SGPA/DGVS/04751/08), as well as the Municipal of Tacotalpa (SM/1133/208), kindly provided permits for the work in Mexico. Financial support came from NSF (DEB-0813783 and DEB-0743406) and DFG (PL 470/1-2). This work represents partial fulfillment of the Ph.D. requirements for R. Riesch.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary Table 1
Sample locations and sample dates. In the main manuscript, the two sites Arroyo Bonita and Río Amatan are pooled and analyzed together as Río Oxolotán sites. N = ratio of “pregnant females to total collected (and dissected) females” (DOCX 12 kb)
Supplementary Table 2
Univariate results from the MANCOVA on female life histories of four different species of poeciliids from two different genera and three levels of evolutionary exposure to toxicity. SL = standard length. All dependent variables and covariates were log-transformed (or arcsine-transformed if percentages) to accommodate for a potential nonlinear relationship between the variables. Only significant results are shown. Adjusted R 2 = 0.975 (DOCX 14 kb)
Supplementary Table 3
Structure matrix and test statistics for DFA on the life-history traits of female poeciliids based on differences in toxicity exposure and genus (DOCX 12 kb)
Supplementary Table 4
Structure matrices and test statistics for DFA on the life-history traits of female poeciliids from habitats with different levels of evolutionary exposure to toxicity (left) and toxic vs nontoxic habitats (right) (DOCX 12 kb)
Supplementary Table 5
Component matrix for the PCA on the life-history traits of female poeciliids from three different levels of evolutionary exposure to toxicity and two genera (DOCX 11 kb)
Supplementary Fig. 1
Group centroids ± SDs of DFAs of female life-history traits based on separation on toxicity exposure alone while controlling for female size (SL) and embryo stage. Nontoxic: (○) P. mexicana and G. sexradiata. Incipient toxic: (●) P. mexicana and G. sexradiata. (●) P. sulphuraria and G. eurystoma (DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Riesch, R., Plath, M., García de León, F.J. et al. Convergent life-history shifts: toxic environments result in big babies in two clades of poeciliids. Naturwissenschaften 97, 133–141 (2010). https://doi.org/10.1007/s00114-009-0613-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-009-0613-y