Abstract
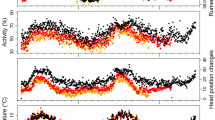
Biological rhythms are a result of interplay between endogenous clocks and the ambient light–dark (LD) cycle. Biological timing in resident polar organisms presents a conundrum because these experience distinct daily LD cycles for only a few weeks each year. We measured locomotor activity in reindeer, Rangifer tarandus platyrhynchus (SR, n = 5 and 6) and R. tarandus tarandus (NR, n = 6), ranging freely at 78 and 70°N, respectively, continuously throughout 1 year using data loggers. NR, but not SR, are gregarious which enabled us to examine the integrated effects of differences in social organisation and the photic environment at two different latitudes on the organisation of activity. In both sub-species, ultradian bouts of activity and inactivity alternated across the 24-h day throughout the year. This pattern was modified by the LD cycle in NR but barely at all in SR. Periodogram analysis revealed significant ultradian rhythmicity in both sub-species; the frequency of daily cycles of activity increased from three per day in winter to nearly five in summer. We conclude that this increase, and a concomitant increase in the level of daily activity, reflected the seasonal increase in the animals’ appetite and the quality of their forage. Secondly, the combination, most evident in SR, of a weak photic response, weak circadian mechanisms and a weak social synchronization reduces the constraints of biological timing in an environment which is effectively non-rhythmic most of the year and permits expression of the basic ultradian pattern of ruminant activity. Third, the weaker 24-h rhythmicity in SR compared to NR indicates a latitudinal decrease in circadian organization and photic responsiveness in Rangifer.





Similar content being viewed by others
References
Andersson H, Johnston JD, Messager S, Hazlerigg D, Lincoln G (2005) Photoperiod regulates clock gene rhythms in the ovine liver. Gen Comp Endcrinol 142:357–363
Aschoff J, Daan S, Honma K-I (1982) Zeitgebers, entrainment and masking: some unsettled questions. In: Ashoff J, Daan S, Groos G (eds) Vertebrate circadian systems: structure and physiology. Springer, Berlin Heidelberg New York, pp 13–24
Barry TN, Suttie JM, Milne JA, Kay RNB (1991) Control of food intake in domesticated deer. In: Tsuda T, Sasaki Y, Kawashima R (eds) Physiological aspects of digestion and metabolism in ruminants. Academic, San Diego, CA, pp 385–401
Boertje RD (1985) An energy model for adult female caribou of the Denali herd, Alaska. J Range Manage 38:468–473
Collins WB, Smith TS (1989) Twenty-four hour behavior patterns and budgets of free-ranging reindeer in winter. Rangifer 9:2–8
Daan S, Aschoff J (2001) Entrainment of circadian rhythms. In: Takahashi JS, Turek FW, Moore RY (eds) Circadian clocks. Plenum, New York, pp 7–43
Davidson AJ, Menaker M (2003) Birds of a feather clock together—sometimes: social synchronization of circadian rhythms. Curr Opin Neurobiol 13:765–769
Dehority BA, Tirabasso PA (2001) Effect of feeding frequency on bacterial and fungal concentrations, pH, and other parameters in the rumen. J Anim Sci 79:2908–2912
Dörrscheidt GL, Beck L (1975) Advanced methods for evaluating characteristic parameters (alpha, tau, rho) of circadian rhythms. J Math Biol 2:107–121
Ebling FJ, Lincoln GA, Wollnik F, Anderson N (1988) Effects of constant darkness and constant light on circadian organization and reproductive responses in the ram. J Biol Rhythms 3:365–384
Eriksson LO, Kallqvist ML, Mossing T (1981) Seasonal development of circadian and short-term activity in captive reindeer, Rangifer tarandus. Oecologia 48:64–70
Gerkema MP, Daan S, Wilbrink, M, Hop MW, van der Leest F (1993) Phase control of ultradian feeding rhythms in the common vole (Microtus arvalis): the roles of light and the circadian system. J Biol Rhythms 8:151–171
Gerkema MP, van der Zee EA, Feitsma L (1994) Expression of circadian rhythmicity correlates with the number of arginine–vasopressin immunoreactive cells in the suprachiasmatic nucleus of common voles, Microtus arvalis. Brain Res 639:93–101
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants—a comparative view of their digestive-system. Oecologia 78:443–457
Hoogenboom I, Daan S, Dallinga JH, Schoenmakers M (1984) Seasonal change in the daily timing of behaviour in the common vole, Microtus arvalis. Oecologia 61:18–31
Hudson RJ (1985) Body size, energetics, and adaptive radiation. In: Hudson RJ, White RG (eds) Bioenergetics of wild herbivores. CRC Press, Boca Raton, FL, pp 1–24
King DP, Takahashi JS (2000) Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci 23:713–742
Klein DR (1990) Variation in quality of caribou and reindeer forage plants associated with season, plant part and phenology. Rangifer Spec Issue 3:123–130
Larsen TS, Nilsson NÖ, Blix AS (1985) Seasonal changes in lipogenesis and lipolysis in isolated adipocytes from Svalbard and Norwegian reindeer. Acta Physiol Scand 123:97–104
Larter NC, Nagy JA (2001) Seasonal and annual variability in the quality of important forage plants on Banks Island, Canadian high Arctic. Appl Veg Sci 4:115–128
Leader-Williams N (1979) Age determination of reindeer introduced into South Georgia. J Zool (Lond) 188:501–515
Leader-Williams N, Ricketts C (1982) Seasonal and sexual patterns of growth and condition of reindeer (Rangifer tarandus) introduced into South Georgia. Oikos 38:27–39
Leedle JAZ, Bryant MP, Hespell RB (1982) Diurnal variations in bacterial numbers and fluid parameters in ruminal contents of animals fed low-forage or high-forage diets. Appl Environ Microbiol 44:402–412
Maier JAK, White RG (1998) Timing and synchrony of activity in caribou. Can J Zool 76:1999–2009
Mathiesen SD, Orpin CG, Greenwood Y, Blix AS (1987) Seasonal changes in the cecal microflora of the high Arctic Svalbard reindeer (Rangifer tarandus platyrhynchus). Appl Environ Microbiol 53:114–118
Mathiesen SD, Sørmo W, Haga Ø, Norberg HJ, Utsi THA, Tyler NJC (2000) The oral anatomy of Arctic ruminants: coping with seasonal changes. J Zool (Lond) 251:119–128
Mesteig K, Tyler NJC, Blix AS (2000) Seasonal changes in heart rate and food intake in reindeer (Rangifer tarandus tarandus). Acta Physiol Scand 170:145–151
Metz JHM (1975) Time patterns of feeding and rumination in domestic cattle. Mededelingen Landbouwhogeschool, Wageningen, No. 75-12, 66 pp
Miller FL (1974) Biology of the Kaminuriak population of barren-ground caribou. Part 2: dentition as an indicator of age and sex: composition and socialisation of the population. Canadian Wildlife Series Report Series No. 31, Ottawa, p 88
Mistlberger RE, Skene DJ (2004) Social influence on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc 79:533–556
Moore-Ede MC, Sulzman FM, Fuller CA (1982) The clocks that time us. Harvard University Press, Cambridge, MA
Nilssen KJ, Johnsen HK, Rognmo A, Blix AS (1984) Heart rate and energy expenditure in resting and running Svalbard and Norwegian reindeer. Am J Physiol 246:963–967
Nyholm ES (1976) Monthly general rhythm pattern variations in the Nordenskiöld Land–Sabine Land population of the Svalbard reindeer (Rangifer tarandus platyrhynchus). Norsk Polarinst Årbok 1974:129–138
Orpin CG, Mathiesen SD, Greenwood Y, Blix AS (1985) Seasonal changes in the ruminal microflora of the high-Arctic Svalbard reindeer (Rangifer tarandus platyrhynchus). Appl Environ Microbiol 50:144–151
Reimers E, Klein DR, Sørumgaard R (1983) Calving time, growth rate, and body size of Norwegian reindeer on different ranges. Arct Alp Res 15:107–118
Roby DD (1980) Winter activity of caribou on two Arctic ranges. In: Reimers E, Gaare E, Skjenneberg S (eds) Proceedings of the 2nd international reindeer/caribou symposium, Røros, Norway, 1979. Direktoratet for vilt og ferskvannsfisk, Trondheim, pp 537–544
Roby DD, Thing H (1985) Behaviour of West Greenland caribou during a population decline. Holarct Ecol 8:77–87
Russell DE, Martell AM, Nixon WAC (1993) Range ecology of the Porcupine caribou head. Rangifer Spec Issue 8:1–168
Skogland T (1984) Wild reindeer foraging niche organization. Holarct Ecol 7:345–379
Skogland T (1989) Comparative social organization of wild reindeer in relation to food, mates and predator avoidance. Adv Ethol 29:1–74
Sørmo W, Haga ØE, White, RG, Mathiesen SD (1997) Comparative aspects of volatile fatty acid production in the rumen and distal fermentation chamber in Svalbard reindeer. Rangifer 17:81–95
Sørmo W, Haga ØE, Gaare E, Langvatn R, Mathiesen SD (1999) Forage chemistry and fermentation chambers in Svalbard reindeer (Rangifer tarandus platyrhynchus). J Zool (Lond) 247:247–256
SPSS (1998) SPSS for Windows Release 9.0.0. SPSS, Chicago, IL
Stokkan KA, Tyler NJC, Reiter RJ (1994) The pineal gland signals autumn to reindeer Rangifer tarandus tarandus) exposed to the continuous daylight of the Arctic summer. Can J Zool 72:904–909
Storeheier PV, Mathiesen SD, Tyler NJC, Schjelderup I, Olsen MA (2002) Utilization of nitrogen- and mineral-rich vascular forage plants by reindeer in winter. J Agric Sci 139:1–10
Storeheier PV, van Oort BEH, Sundset MA, Mathiesen SD (2003) Food intake of reindeer in winter. J Agric Sci 141:93–101
Tyler NJC (1987) Body composition and energy balance of pregnant and non-pregnant Svalbard reindeer during winter. Symp Zool Soc Lond 57:203–229
Tyler NJC, Øritsland NA (1999) Varig utstabilitet og bestandsregulering hos svalbardrein. [Persistant instability and populations regulation in Svalbard reindeer]. Norsk Polarinst Medd 150:125–138
Tyler NJC, Fauchald P, Johansen O, Christiansen HR (1999) Seasonal inappetence and weight loss in female reindeer in winter. Ecol Bull 47:105–116
van Oort BEH, Tyler NJC, Storeheier PV, Stokkan K-A (2004) The performance and validation of a data logger for long-term determination of activity in free-ranging reindeer, Rangifer tarandus (L.) Appl Anim Behav Sci 89:299–308
van Oort BEH, Tyler NJC, Gerkema MP, Folkow L, Blix AS, Stokkan K-A (2005) Circadian organization in reindeer. Nature 438:1095–1096
Vrolik W (1829) Over eene vermoedelijke tweede soort van Rendier. Nieuwe Verh Kon Nederl Inst Eerst Klass Pt 2:153–160
White RG, Staaland H (1983) Ruminal volatile fatty acid production as an indicator of forage quality in Svalbard reindeer. Acta Zool Fenn 175:61–63
Acknowledgements
We thank reindeer herder J.H. Eira for his cooperation during the project. The study was partly supported by the Research Council of Norway grant number 121526/720 and the Reindeer Husbandry Research Fund. B.E.H. van Oort was a recipient of a TMR Marie Curie Research Training Grant (#ERB4001GT964370).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Oort, B.E.H., Tyler, N.J.C., Gerkema, M.P. et al. Where clocks are redundant: weak circadian mechanisms in reindeer living under polar photic conditions. Naturwissenschaften 94, 183–194 (2007). https://doi.org/10.1007/s00114-006-0174-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-006-0174-2