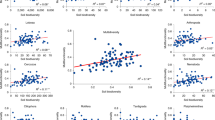
Abstract
The European soil policy is being focussed towards a more conscious and sustainable use of the soil, taking into account ecological, economical and societal dimensions. Living soil organisms are reliable bioindicators, as they provide the best reflection of the soil system, ecological services and ecosystem functioning therein. These most complex (bio)physical systems indicate, among others, the energy flow. Such processes can be described by rather simple power law relationships. In fact, the average body mass (dry weight) can be seen as an inherent species property, while population density is a much more flexible parameter reflecting ecosystem state. In this study, I review the interactions between these items in relation to feedbacks and conjectured relationships which can be seen as ecological networks. From this novel perspective, allometry can be used as an integrated measure for the anthropogenic influence on landscapes and related food webs. Allometry is, therefore, a perfect surrogate for land use intensity in modelling of field effects for restoration ecology and conservation biology. Robust correlations will be addressed between the density dependence of invertebrates and the ability of soil systems themselves to recover after disturbance. Quantitative indicators of soil community composition and related ecological services are proposed and their application for ecological risk assessment is illustrated.



Similar content being viewed by others
References
Allen AP, Gillooly JF, Brown JH (2005) Linking the global carbon cycle to individual metabolism. Funct Ecol 19:202–213
Ashman MR, Hallett PD, Brookes PC (2003) Are the links between soil aggregate size class, soil organic matter and respiration rate artefacts of the fractionation procedure? Soil Biol Biochem 35:435–444
Bardgett RD (2002) Causes and consequences of biological diversity in soil. Zoology 105:367–374
Bardgett RD, Wardle DA, Yeates GW (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 30:1867–1878
Bardgett RD, Cook R, Yeates GW, Denton CS (1999) The influence of nematodes on below-ground processes in grassland ecosystems. Plant Soil 212:23–33
Bardgett RD, Streeter TC, Bol R (2003) Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 84:1277–1287
Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK (2005) A temporal approach to linking aboveground and belowground ecology. Trends Ecol Evol 20:634–641
Beerling DJ, Woodward FI (2001) Vegetation and the terrestrial carbon cycle: modelling the first 400 million years. Cambridge University Press, Cambridge
Begon M, Townsend CR, Harper JL (2006) Ecology: from individuals to ecosystems. Blackwell, Oxford
Bengtsson J (1998) Which species? What kind of diversity? Which ecosystem function? Some problems in studies of relations between biodiversity and ecosystem function. Appl Soil Ecol 10:191–199
Bengtsson J, Persson T, Lundkvist H (1997) Long-term effects of logging residue addition and removal on macroarthropods and enchytraeids. J Appl Ecol 34:1014–1022
Bengtsson J, Lundkvist H, Saetre P, Sohlenius B, Solbreck B (1998) Effects of organic matter removal on the soil food web: forestry practices meet ecological theory. Appl Soil Ecol 9:137–143
Bengtsson J, Engelhardt K, Giller P, Hobbie S, Lawrence D, Levine J, Vilà M, Wolters V (2002) Slippin’ and slidin’ between the scales: the scaling components of biodiversity–ecosystem functioning relations. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, Oxford, pp 209–220
Bengtsson J, Ahnström J, Weibull A-C (2005) The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J Appl Ecol 42:261–269
Berryman AA (1991) Stabilization or regulation: what it all means! Oecologia 86:140–143
Bever JD (2003) Soil community feedback and the coexistence of competitors: conceptual framework and empirical tests. New Phytol 157:465–473
Bonkowski M (2004) Protozoa and plant growth: the microbial loop in soil revisited. New Phytol 162:617–631
Bradford MA, Jones TH, Bardgett RD, Black HIJ, Boag B, Bonkowski M, Cook R, Eggers T, Gange AC, Grayston SJ et al (2002) Impacts of soil faunal community composition on model grassland ecosystems. Science 298:615–618
Breure AM (2004) Ecological soil monitoring and quality assessment. In: Doelman P, Eijsackers HJP (eds) Vital soil: function, value and properties. Developments in soil science, vol 29. Elsevier Science, Amsterdam, pp 281–305
Breure AM, Mulder C, Römbke J, Ruf A (2005) Ecological classification and assessment concepts in soil protection. Ecotoxicol Environ Saf 62:211–229
Brown GG (1995) How do earthworms affect microfloral and faunal community diversity? Plant Soil 170:209–231
Cain M, Milligan B, Strand A (2000) Long-distance seed dispersal in plant populations. Am J Bot 87:1217–1227
Cairney JWG (2000) Evolution of mycorrhiza systems. Naturwissenschaften 87:467–475
Chapin FS III, Eviner VT (2003) Biogeochemistry of terrestrial net primary production. In: Schlesinger WH (ed) Treatise on geochemistry, vol 8. Biogeochemistry. Elsevier, pp 215-247
Cohen JE (1994) Marine and continental food webs: three paradoxes? Philos Trans R Soc Lond B 343:57–69
Cohen JE, Jonsson T, Carpenter SR (2003) Ecological community description using the food web, species abundance, and body size. Proc Natl Acad Sci USA 100:1781–1786
Cole L, Staddon PL, Sleep D, Bardgett RD (2004) Soil animals influence microbial abundance, but not plant–microbial competition for soil organic nitrogen. Funct Ecol 18:631–640
Coleman DC, Reid CPP, Cole CV (1983) Biological strategies of nutrient cycles in soil systems. Adv Ecol Res 13:1–55
Cook BD, Allan DL (1992) Dissolved organic matter in old field soils: total amounts as a measure of available resources for soil mineralization. Soil Biol Biochem 24:585–594
Cousins SH (1980) A trophic continuum derived from plant structure, animal size and a detritus cascade. J Theor Biol 82:607–618
Cousins SH (1987) The decline of the trophic level concept. Trends Ecol Evol 2:312–316
Cox CB, Moore PD (2005) Biogeography: an ecological and evolutionary approach. Blackwell, Oxford
Damuth J (1981) Population density and body size in mammals. Nature 290:699–700
Dawson LA, Grayston SJ, Paterson E (2000) Effects of grazing on the roots and rhizosphere of grasses. In: Lemaire G, Hodgson J, De Moraes A, Nabinger C, De F Carvalho PC (eds) Grassland ecophysiology and grazing ecology. CAB International, Wallingford, Oxon, UK, pp 61–84
De Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29:795–811
De Deyn GB, Van der Putten WH (2005) Linking aboveground and belowground diversity. Trends Ecol Evol 20:625–633
De Luca TH, Keeney DR (1993) Soluble anthrone-reactive carbon in soils: effect of carbon and nitrogen amendments. Soil Sci Soc Am J 57:1296–1300
Del Monte-Luna P, Brook BW, Zetina-Rejón MJ, Cruz-Escalona VH (2004) The carrying capacity of ecosystems. Glob Ecol Biogeogr 13:485–495
Dennis RLH, Hodgson JG, Grenyer R, Shreeve TG, Roy DB (2004) Host plants and butterfly biology. Do host-plants strategies drive butterfly status? Ecol Entomol 29:12–26
Dennis RLH, Shreeve TG, Arnold HR, Roy DB (2005) Does diet breadth control herbivorous insect distribution size? Life history and resource outlets for specialist butterflies. J Insect Conserv 9:187–200
Didden W (2003) Oligochaeta. In: Markert B, Breure AM, Zechmeister H (eds) Bioindicators and biomonitors. Elsevier Science and Technology, London, pp 555–576
Didden W, Fründ HC, Graefe U (1997) Enchytraeids. In: Benckiser GE (ed) Fauna in soil ecosystems: recycling processes, nutrient fluxes and agricultural production. Marcel Dekker, New York, pp 135–172
Dunne JA (2005) The network structure of food webs. In: Pascual M, Dunne JA (eds) Ecological networks: linking structure to dynamics in food webs. Oxford University Press, Oxford, pp 27–86
Ebenman B, Jonsson T (2005) Using community viability analysis to identify fragile systems and keystone species. Trends Ecol Evol 20:568–575
Farley RA, Fitter AH (1999) Temporal and spatial variation in soil resources in a deciduous woodland. J Ecol 87:688–696
Filser J (2002) The role of Collembola in carbon and nitrogen cycling in soil. Pedobiologia 46:234–245
Filser J, Mebes K-H, Winter K, Lang A, Kampichler C (2002) Long-term dynamics and interrelationships of soil Collembola and microorganisms in an arable landscape following land use change. Geoderma 105:201–222
Fitter AH, Sanders I (1992) Interactions with the soil fauna. In: Allen MF (ed) Mycorrhizal functioning. Chapman & Hall, New York, pp 333–354
Fitter AH, Gilligan CA, Hollingworth K, Kleczkowski A, Twyman RM, Pitchford JW (2005) Biodiversity and ecosystem function in soil. Funct Ecol 19:369–377
Fontaine S, Barot S (2005) Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol Lett 8:1075–1087
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320
Fountain MT, Hopkin SP (2005) Folsomia candida (Collembola): a “standard” soil arthropod. Annu Rev Entomol 50:201–222
Gange AC (1993) Translocation of mycorrhizal fungi by earthworms during early plant succession. Soil Biol Biochem 25:1021–1026
Gange AC (2000) Species-specific responses of a root- and shoot-feeding insect to arbuscular mycorrhizal colonization of its host plant. New Phytol 150:611–618
Gange AC, Brown VK (2002) Actions and interactions of soil invertebrates and arbuscular mycorrhizal fungi in affecting the structure of plant communities. Ecol Stud 157:321–344
Gange AC, Stagg PG, Ward LK (2002) Arbuscular mycorrhizal fungi affect phytophagous insect specialism. Ecol Lett 5:11–15
Gange AC, Brown VK, Aplin DM (2005) Ecological specificity of arbuscular mycorrhizae: evidence from foliar- and seed-feeding insects. Ecology 86:603–611
Giller KE, Beare MH, Lavelle P, Izac AMN, Swift MJ (1997) Agricultural intensification, soil biodiversity and agro-ecosystem function. Appl Soil Ecol 6:3–16
Griffiths BS, Ritz R, Bardgett RD, Cook R, Christensen S, Ekelund F, Sørensen E, Bååth E, Bloem J, De Ruiter P et al (2000) Stability of soil ecosystem-level processes following the experimental manipulation of soil microbial community diversity. Oikos 90:279–294
Hammer K, Arrowsmith N, Gladis T (2003) Agrobiodiversity with emphasis on plant genetic resources. Naturwissenschaften 90:241–250
Hartnett DC, Wilson GWT (1999) Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80:1187–1195
Hedlund K, Griffiths B, Christensen S, Scheu S, Setälä H, Tscharntke T, Verhoef H (2004) Trophic interactions in changing landscapes: responses of soil food webs. Basic Appl Ecol 5:495–503
Hendrix PF, Crossley DA Jr, Blair JM, Coleman DC (1990) Soil biota as components of sustainable agroecosystems. In: Edwards CA, Lal R, Madden P, Miller RH, House G (eds) Sustainable agricultural systems. Soil and Water Conservation Society, Ankeny, IL, pp 637–654
Högberg MN, Högberg P (2002) Extramatrical ectomycorrhizal mycelium contributes half the microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol 154:791–795
Holling CS (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–23
Holling CS (1992) Cross-scale morphology, geometry and dynamics of ecosystems. Ecol Monogr 62:447–502
Hunt HW, Coleman DC, Ingham ER, Ingham RE, Elliot ET, Moore JC, Rose SL, Reid CPP, Morley CR (1987) The detrital food web in a shortgrass prairie. Biol Fertil Soils 3:57–68
Ingham RE, Trofymow JA, Ingham ER, Coleman DC (1985) Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol Monogr 55:119–140
Ives AR, Gross K, Klug JL (1999) Stability and variability in competitive communities. Science 286:542–544
Ives AR, Klug JL, Gross K (2000) Stability and species richness in complex communities. Ecol Lett 3:399–411
Jansa J, Mozafar A, Anken T, Ruh R, Sanders IR, Frossaard E (2002) Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza 12:225–234
Jonsson T, Cohen JE, Carpenter SR (2005) Food webs, body size and species abundance in ecological community description. Adv Ecol Res 36:1–84
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Klironomos JN, Kendrick WB (1995) Stimulative effects of arthropods on endomycorrhizas of sugar maple in the presence of decaying litter. Funct Ecol 9:528–536
Klironomos JN, Kendrick WB (1996) Palatability of microfungi to soil arthropods in relation to the functioning of arbuscular mycorrhizae. Biol Fertil Soils 21:43–52
Klironomos JN, Hart MM (2001) Animal nitrogen swap for plant carbon. Nature 410:651–652
Klironomos JN, Bednarczuk EM, Neville J (1999) Reproductive significance of feeding on saprobic and arbuscular mycorrhizal fungi by the collembolan, Folsomia candida. Funct Ecol 13:756–761
Klironomos JN, McCune J, Hart M, Neville J (2000) The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecol Lett 3:137–141
Laakso J, Setälä H (1999) Sensitivity of primary production to changes in the architecture of belowground food webs. Oikos 87:57–64
Laakso J, Setälä H, Palojärvi A (2000) Influence of decomposer food web structure and nitrogen availability on plant growth. Plant Soil 225:153–165
Legendre L, Rivkin RB (2002) Pelagic food webs: responses to environmental processes and effects on the environment. Ecol Res 17:143–149
Liiri M, Setälä H, Haimi J, Pennanen T, Fritze H (2002) Relationship between soil microarthropod species diversity and plant growth does not change when the system is disturbed. Oikos 96:137–149
Loreau M, Downing A, Emmerson M, Gonzalez A, Hughes J, Inchausti P, Joshi J, Norberg J, Sala O (2002) A new look at the relationship between diversity and stability. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, Oxford, pp 79–91
Marquet PA, Quiñones RA, Abades S, Labra F, Tognelli M, Arim M, Rivadeneira M (2005) Scaling and power-laws in ecological systems. J Exp Biol 208:1749–1769
Melián CJ, Bascompte J (2002) Food web structure and habitat loss. Ecol Lett 5:37–46
Meyerson LA, Baron J, Melillo JM, Naiman RJ, O’Malley RI, Orians G, Palmer MA, Pfaff ASP, Running SW, Sala OE (2005) Aggregate measures of ecosystem services: can we take the pulse of nature? Front Ecol Environ 3:56–59
Mikola J, Bardgett RD, Hedlund K (2002) Biodiversity, ecosystem functioning and soil decomposer food webs. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, Oxford, pp 169–180
Moore JC, Hunt HW (1988) Resource compartmentation and the stability of real ecosystems. Science 261:906–909
Moore JC, Walter DE, Hunt HW (1988) Arthropod regulation of micro- and mesobiota in below-ground detrital food web. Annu Rev Entomol 33:419–439
Moore JC, Berlow EL, Coleman DC, De Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ et al (2004) Detritus, trophic dynamics and biodiversity. Ecol Lett 7:584–600
Mulder C, Janssen CR (1999) Occurrence of pollen and spores in relation to present-day vegetation in a Dutch heathland area. J Veg Sci 10:87–100
Mulder C, De Zwart D (2003) Assessing fungal species sensitivity to environmental gradients by the Ellenberg indicator values of above-ground vegetation. Basic Appl Ecol 4:557–568
Mulder C, Sakorafa V, Burragato F, Visscher H (2000) Ecohydrological perspective of phytogenic organic and inorganic components in Greek lignites: a quantitative reinterpretation. Earth Planet Sci Lett 179:167–181
Mulder C, De Zwart D, Van Wijnen HJ, Schouten AJ, Breure AM (2003a) Observational and simulated evidence of ecological shifts within the soil nematode community of agroecosystems under conventional and organic farming. Funct Ecol 17:516–525
Mulder C, Breure AM, Joosten JHJ (2003b) Fungal functional diversity inferred along Ellenberg’s abiotic gradients: palynological evidence from different soil microbiota. Grana 42:55–64
Mulder C, Van Wijnen HJ, Van Wezel AP (2005a) Numerical abundance and biodiversity of below-ground taxocenes along a pH gradient across the Netherlands. J Biogeogr 32:1775–1790
Mulder C, Aldenberg T, De Zwart D, Van Wijnen HJ, Breure AM (2005b) Evaluating the impact of pollution on plant–Lepidoptera relationships. Environmetrics 16:357–373
Mulder C, Cohen JE, Setälä H, Bloem J, Breure AM (2005c) Bacterial traits, organism mass, and numerical abundance in the detrital soil food web of Dutch agricultural grasslands. Ecol Lett 8:80–90
Mulder C, Dijkstra JB, Setälä H (2005d) Nonparasitic Nematoda provide evidence for a linear response of functionally important soil biota to increasing livestock density. Naturwissenschaften 92:314–318
Mulder C, Van Wezel AP, Van Wijnen HJ (2005e) Embedding soil quality in the planning and management of land use. Int J Biodiv Sci Manag 1:77–84
Needham SJ, Worden RH, McIlroy D (2004) Animal–sediment interactions: the effect of ingestion and excretion by worms on mineralogy. Biogeosciences 1:113–121
Nicholson AJ (1933) The balance of animal populations. J Anim Ecol 2:132–178
Nicholson AJ (1954) An outline of the dynamics of animal populations. Aust J Zool 2:9–65
Nicholson AJ, Bailey VA (1935) The balance of animal populations. Part I. Proc Zool Soc Lond 3:551–598
O’Connell T, Bolger T (1997) Fungus fruiting bodies and the structure of fungus–microarthropod assemblages. Proc R Ir Acad 97B:249–262
Perez-Moreno J, Read DJ (2001) Nutrient transfer from soil nematodes to plants: a direct pathway provided by the mycorrhizal mycelial network. Plant Cell Environ 24:1219–1226
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–492
Reuman DC, Cohen JE (2004) Trophic links’ length and slope in the Tuesday Lake food web with species’ body mass and numerical abundance. J Anim Ecol 73:52–866
Reuman DC, Cohen JE (2005) Estimating relative energy fluxes using the food web, species abundance, and body size. Adv Ecol Res 36:137–182
Reuman DC, Mulder C, Banašek-Richter C, Cattin Blandenier M-F, Breure AM, Den Hollander HA, Kneitel JM, Raffaelli D, Woodward G, Cohen JE (2006) Allometry of body size and abundance in 166 food webs: tests of the standard linear model. Rockefeller University
Römbke J, Jänsch S, Didden W (2005) The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicol Environ Saf 62:266–277
Scheu S, Setälä H (2002) Multitrophic interactions in decomposer food-webs. In: Tscharntke T, Hawkins BA (eds) Multitrophic level interactions. Cambridge University Press, Cambridge, pp 223–264
Setälä H (1995) Growth of birch and pine seedlings in relation to grazing by soil fauna on ectomycorrhizal fungi. Ecology 76:1844–1851
Setälä H (2000) Reciprocal interactions between Scots pine and soil food web structure in the presence and absence of mycorrhiza. Oecologia 125:109–118
Setälä H (2002) Sensitivity of ecosystem functioning to changes in trophic structure, functional group composition and species diversity in belowground food webs. Ecol Res 17:207–215
Setälä H, Laakso J, Mikola J, Huhta V (1998) Functional diversity of decomposer organisms in relation to primary production. Appl Soil Ecol 9:25–31
Siemann E, Tilman D, Haarstad J (1999) Abundance, diversity and body size: patterns from a grassland arthropod community. J Anim Ecol 68:824–835
Solé RV, Ferrer-Cancho R, Montoya JM, Valverde S (2003) Selection, tinkering, and emergence in complex networks: crossing the land of tinkering. Complexity 8:20–33
Sultan SE, Spencer HG (2002) Metapopulation structure favors plasticity over local adaptation. Am Nat 160:271–283
Van Noordwijk M, Martikainen P, Bottner P, Cuevas E, Rouland C, Dhillion SS (1998) Global change and root function. Glob Chang Biol 4:759–772
Walker DW, McColl G, Jenkins NL, Harris J, Lithgow GJ (2000) Evolution of lifespan in C. elegans. Nature 405:296–297
Wardle DA (1995) Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices. Adv Ecol Res 26:105–185
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Monographs in population biology 34. Princeton University Press
Wardle DA, Yeates GW (1993) The dual importance of competition and predation as regulatory forces in terrestrial ecosystems: evidence from decomposer food-webs. Oecologia 93:303–306
Wardle DA, Bonner KI, Barker GM, Yeates GW, Nicholson KS, Bardgett RD, Watson RN, Ghani A (1999) Plant removals in perennial grassland: vegetation dynamics, decomposers, soil biodiversity, and ecosystem properties. Ecol Monogr 69:535–568
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, Van der Putten W, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Warwick RM, Clarke KR (1998) Taxonomic distinctness and environmental assessment. J Appl Ecol 35:532–543
Watkinson AR, Freckleton RP (1997) Quantifying the impact of arbuscular mycorrhiza on plant competition. J Ecol 85:541–546
Werger MJA (1978) Biogeography and ecology of Southern Africa. Junk, The Hague
West GB, Brown JH (2005) The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol 208:1575–1592
West GB, Woodruff WH, Brown JH (2002) Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Natl Acad Sci USA 99:2473–2478
Woodward G, Ebenman B, Emmerson M, Montoya JM, Olesen JM, Valido A, Warren PH (2005) Body size in ecological networks. Trends Ecol Evol 20:402–409
Yeates GW (2003) Nematodes as soil indicators: functional and biodiversity aspects. Biol Fertil Soils 37:199–210
Yeates GW, Saggar S, Denton CS, Mercer CF (1998) Impact of clover cyst nematode (Heterodera trifolii) infection on soil microbial activity in the rhizosphere of white clover (Trifolium repens): a pulse labelling experiment. Nematologica 44:81–90
Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS (1993) Feeding habits in soil nematode families and genera—an outline for soil ecologists. J Nematol 25:315–331
Zolda P (2006) Nematode communities of grazed and ungrazed semi-natural steppe grasslands in Eastern Austria. Pedobiologia 50:11–22
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulder, C. Driving forces from soil invertebrates to ecosystem functioning: the allometric perspective. Naturwissenschaften 93, 467–479 (2006). https://doi.org/10.1007/s00114-006-0130-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-006-0130-1