Abstract
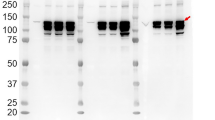
Soluble guanylyl cyclase (sGC) is the main receptor for nitric oxide, a messenger molecule with multiple clinical implications. Understanding the activation of sGC is an important step for establishing new therapeutic principles. We have now overexpressed sGC in a baculovirus/Sf9 system optimized for high protein yields to facilitate spectral and kinetic studies of the activation mechanisms of this enzyme. It was expressed in a batch fermenter using a defined mixture of viruses encoding the α1 and β1 subunits of the rat lung enzyme. The expressed enzyme was purified from the cytosolic fraction by anion exchange chromatography, hydroxyapatite chromatography, and size exclusion chromatography. By use of this new method 2.5 l culture yielded about 1 mg of apparently homogeneous sGC with a content of about one heme per heterodimer without the need of a heme reconstitution step. The enzyme did not contain stoichiometric amounts of copper. The basal activities of the purified enzyme were 153 and 1259 nmol min–1 mg–1 in the presence of Mg2+ and Mn2+, respectively. The nitric oxide releasing agent 2-(N,N-diethylamino)-diazenolate-2-oxide (DEA/NO) stimulated the enzyme 160-fold with Mg2+, whereas the NO-independent activator 3-(5’-hydroxymethyl-2’-furyl)-1- benzylindazole (YC-1) induced an increase in the activity of 101-fold at a concentration of 300 µM. The combination of DEA/NO (10 µM) and YC-1 (100 µM) elicited a dose-dependent synergistic stimulation with a maximum of a 792-fold increase over the basal activity in the presence of Mg2+, resulting in a specific activity of 121 µmol min–1 mg–1. The synergistic stimulation of DEA/NO and YC-1 was attenuated by the sGC inhibitor 1H-(1,2,4)oxadiazole(4,3-a)quinoxalin-1-one (ODQ) (10 µM) by 94%. In a different experimental setup a saturated carbon monoxide solution in the absence of ambient oxygen or NO stimulated the enzyme 15-fold in the absence and 1260-fold in the presence of YC-1 compared to an argon control. The heme spectra of the enzyme showed a shift of the Soret peak from 432 to 399 and 424 nm in the presence of DEA/NO or carbon monoxide, respectively. The heme spectra were not affected by YC-1 in the absence or in the presence of DEA/NO or of carbon monoxide, which reflects the fact that YC-1 does not interact directly with the heme group of the enzyme. In summary, this study shows that our expression/purification procedure is suitable for producing large amounts of highly pure sGC which contains one heme per heterodimer without a reconstitution step. The activator experiments show that in a synergistic stimulation with YC-1 sGC can be activated maximally both by nitric oxide and by carbon monoxide and that YC-1 does not directly act via heme. The described method should help to facilitate the investigation of the new therapeutic principle of NO-independent guanylyl cyclase activators.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 20 May 1998 / Accepted: 5 October 1998
Rights and permissions
About this article
Cite this article
Hoenicka, M., Becker, EM., Apeler, H. et al. Purified soluble guanylyl cyclase expressed in a baculovirus/Sf9 system: stimulation by YC-1, nitric oxide, and carbon monoxide. J Mol Med 77, 14–23 (1999). https://doi.org/10.1007/s001090050292
Issue Date:
DOI: https://doi.org/10.1007/s001090050292