Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrosing interstitial pneumonia of unknown etiology. The role of genetic risk factors has been the focus of numerous studies probing for associations of genetic variants with IPF. We aimed to determine whether single-nucleotide polymorphisms (SNPs) of four candidate genes are associated with IPF susceptibility and survival in a Portuguese population. A retrospective case–control study was performed with 64 IPF patients and 74 healthy controls. Ten single-nucleotide variants residing in the MUC5B, TOLLIP, SERPINB1, and PLAU genes were analyzed. Single- and multi-locus analyses were performed to investigate the predictive potential of specific variants in IPF susceptibility and survival. Multifactor dimensionality reduction (MDR) was employed to uncover predictive multi-locus interactions underlying IPF susceptibility. The MUC5B rs35705950 SNP was significantly associated with IPF: T allele carriers were significantly more frequent among IPF patients (75.0% vs 20.3%, P < 1.0 × 10−6). Genotypic and allelic distributions of TOLLIP, PLAU, and SERPINB1 SNPs did not differ significantly between groups. However, the MUC5B-TOLLIP T-C-T-C haplotype, defined by the rs35705950-rs111521887-rs5743894-rs5743854 block, emerged as an independent protective factor in IPF survival (HR = 0.37, 95% CI 0.17–0.78, P = 0.009, after adjustment for FVC). No significant multi-locus interactions correlating with disease susceptibility were detected. MUC5B rs35705950 was linked to an increased risk for IPF, as reported for other populations, but not to disease survival. A haplotype incorporating SNPs of the MUC5B-TOLLIP locus at 11p15.5 seems to predict better survival and could prove useful for prognostic purposes and IPF patient stratification.
Key messages
-
The MUC5B rs35705950 minor allele is associated with IPF risk in the Portuguese.
-
No predictive multi-locus interactions of IPF susceptibility were identified by MDR.
-
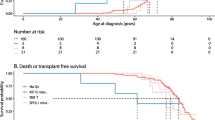
A haplotype defined by MUC5B and TOLLIP SNPs is a protective factor in IPF survival.
-
The haplotype may be used as a prognostic tool for IPF patient stratification.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions, but are available from the corresponding author on reasonable request.
References
Coward WR, Saini G, Jenkins G (2010) The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis 4:367–388. https://doi.org/10.1177/1753465810379801
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ et al (2018) Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 198:e44–68. https://doi.org/10.1164/rccm.201807-1255ST
Selman M, King J, Pardo A (2001) Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134:136–151
Lv M, Liu Y, Ma S, Yu Z (2019) Current advances in idiopathic pulmonary fibrosis: the pathogenesis, therapeutic strategies and candidate molecules. Future Med Chem 11:2595–2620. https://doi.org/10.4155/fmc-2019-0111
Li X, Kim SE, Chen TY, Wang J, Yang X, Tabib T et al (2020) Toll Interacting Protein protects bronchial epithelial cells from bleomycin-induced apoptosis. FASEB J 34:9884. https://doi.org/10.1096/fj.201902636RR
Spagnolo P, Grunewald J, Du Bois RM (2014) Genetic determinants of pulmonary fibrosis: evolving concepts. Lancet Respir Med 2:416–428
Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y et al (2013) Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med 1:309–317. https://pubmed.ncbi.nlm.nih.gov/24429156/
Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP et al (2013) Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet 45:613–620. https://www.nature.com/articles/ng.2609
Allen RJ, Porte J, Braybrooke R, Flores C, Fingerlin TE, Oldham JM et al (2017) Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir Med 5:869–880. https://pubmed.ncbi.nlm.nih.gov/29066090/
Newton CA, Molyneaux PL, Oldham JM (2018) Clinical genetics in interstitial lung disease. Front Med 5:116. https://doi.org/10.3389/fmed.2018.00116
Coghlan MA, Shifren A, Huang HJ, Russell TD, Mitra RD, Zhang Q et al (2014) Sequencing of idiopathic pulmonary fibrosis-related genes reveals independent single gene associations. BMJ Open Respir Res 1:e000057. https://bmjopenrespres.bmj.com/content/1/1/e000057
Petrovski S, Todd JL, Durheim MT, Wang Q, Chien JW, Kelly FL et al (2017) An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. Am J Respir Crit Care Med 196:82–93. https://pubmed.ncbi.nlm.nih.gov/28099038/
Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE et al (2011) A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364:1503–1512. https://pubmed.ncbi.nlm.nih.gov/21506741/
Zhang Y, Noth I, Garcia JGN, Kaminski N (2011) A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med.364:1576–1577. https://doi.org/10.1056/NEJMc1013504
Peljto AL, Zhang Y, Fingerlin TE, Shwu-Fan M, Garcia JGN, Richards TJ et al (2013) Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA 309:2232–2239. https://pubmed.ncbi.nlm.nih.gov/23695349/
Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L et al (2016) Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev 96:1567–1591. https://doi.org/10.1152/physrev.00004.2016
Ramos E, Lopes C, Barros H (2004) Investigating the effect of nonparticipation using a population-based case–control study on myocardial infarction. Ann Epidemiol 14:437–441
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK et al (2011) An Official ATS/ERS/JRS/ALAT Statement: Idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183:788–824. https://doi.org/10.1164/rccm.2009-040GL
Robalo Cordeiro C, Campos P, Carvalho L, Campainha S, Clemente S, Figueiredo L et al (2016) Consensus document for the diagnosis and treatment of idiopathic pulmonary fibrosis: joint consensus of Sociedade Portuguesa de Pneumologia, Sociedade Portuguesa de Radiologia e Medicina Nuclear e Sociedade Portuguesa de Anatomia Patológica. Rev Port Pneumol (English Ed) 22:112–122
Raghu G, Rochwerg B, Zhang Y et al (2015) An Official ATS/ERS/JRS/ALAT Clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline [published correction appears in Am J Respir Crit Care Med. 2015 Sep 1;192(5):644. Dosage error in article text]. Am J Respir Crit Care Med 192:e3–e19. https://doi.org/10.1164/rccm.201506-1063ST
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989
Stephens M, Scheet P (2005) Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet 76:449–462. https://doi.org/10.1086/428594
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265. https://pubmed.ncbi.nlm.nih.gov/15297300/
Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF et al (2001) Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet 69:138–147. https://pubmed.ncbi.nlm.nih.gov/11404819/
Moore JH, Andrews PC (2015) Epistasis analysis using multifactor dimensionality reduction. Methods Mol Biol 1253:301–314. https://doi.org/10.1007/978-1-4939-2155-3_16
Greene CS, Himmelstein DS, Nelson HH, Kelsey KT, Williams SM, Andrew AS et al (2010) Enabling personal genomics with an explicit test of epistasis. Pacific Symp Biocomput 2010:327–336. https://doi.org/10.1142/9789814295291_0035
Moore JH, Hu T (2015) Epistasis analysis using information theory. Methods Mol Biol 1253:257–268. https://doi.org/10.1007/978-1-4939-2155-3_13
Zhu QQ, Zhang XL, Zhang SM, Tang SW, Min HY, Yi L et al (2015) Association between the MUC5B promoter polymorphism rs35705950 and idiopathic pulmonary fibrosis: a meta-analysis and trial sequential analysis in caucasian and asian populations. Medicine (Baltimore) 94:e1901. https://pubmed.ncbi.nlm.nih.gov/26512610/
Stock CJ, Sato H, Fonseca C, Banya WAS, Molyneaux PL, Adamali H et al (2013) Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax 68:436–441. https://thorax.bmj.com/content/68/5/436
Horimasu Y, Ohshimo S, Bonella F, Tanaka S, Ishikawa N, Hattori N et al (2015) MUC5B promoter polymorphism in Japanese patients with idiopathic pulmonary fibrosis. Respirology 20(3):439–444. https://doi.org/10.1111/resp.12466
Peljto AL, Selman M, Kim DS, Murphy E, Tucker L, Pardo A, et al (2015) The MUC5B promoter polymorphism is associated with idiopathic pulmonary fibrosis in a Mexican cohort but is rare among Asian ancestries. Chest 147:460–464. https://pubmed.ncbi.nlm.nih.gov/25275363/
Wang C, Zhuang Y, Guo W, Cao L, Zhang H, Xu L et al (2014) Mucin 5B promoter polymorphism is associated with susceptibility to interstitial lung diseases in Chinese males. PLoS One 9:e104919. https://doi.org/10.1371/journal.pone.0104919
Jiang H, Hu Y, Shang L, Li Y, Yang L, Chen Y (2015) Association between MUC5B polymorphism and susceptibility and severity of idiopathic pulmonary fibrosis. Int J Clin Exp Pathol 8:14953–14958
Van Der Vis JJ, Snetselaar R, Kazemier KM, Ten Klooster L, Grutters JC, Van Moorsel CHM (2016) Effect of Muc5b promoter polymorphism on disease predisposition and survival in idiopathic interstitial pneumonias. Respirology 21:712–717. https://doi.org/10.1111/resp.12728
Bonella F, Campo I, Zorzetto M, Boerner E, Ohshimo S, Theegarten D et al (2021) Potential clinical utility of MUC5B und TOLLIP single nucleotide polymorphisms (SNPs) in the management of patients with IPF. Orphanet J Rare Dis 16(1):1–9. https://doi.org/10.1186/s13023-021-01750-3
Oldham JM, Ma SF, Martinez FJ, Anstrom KJ, Raghu G, Schwartz DA et al (2015) TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 192(12):1475–1482. https://doi.org/10.1164/rccm.201505-1010OC
Auton A, Abecasis GR, Altshuler DM, Durbin RM, Bentley DR, Chakravarti A et al (2015) A global reference for human genetic variation. Nature 526:68–74. https://www.nature.com/articles/nature15393
Shirali M, Knott SA, Pong-Wong R, Navarro P, Haley CS (2018) Haplotype heritability mapping method uncovers missing heritability of complex traits. Sci Reports 8:1–9. https://www.nature.com/articles/s41598-018-23307-4
Schrodi SJ, Garcia VE, Rowland C, Jones HB (2006) Pairwise linkage disequilibrium under disease models. Eur J Hum Genet 15:212–220. https://www.nature.com/articles/5201731
Khankhanian P, Gourraud PA, Lizee A, Goodin DS (2015) Haplotype-based approach to known MS-associated regions increases the amount of explained risk. J Med Genet 52:587–594. https://pubmed.ncbi.nlm.nih.gov/26185143/
Biondini D, Cocconcelli E, Bernardinello N, Lorenzoni G, Rigobello C, Lococo S et al (2021) Prognostic role of MUC5B rs35705950 genotype in patients with idiopathic pulmonary fibrosis (IPF) on antifibrotic treatment. Respir Res 22:98. https://doi.org/10.1186/s12931-021-01694-z
Li X, Goobie GC, Gregory AD, Kass DJ, Zhang Y (2021) Toll-interacting protein in pulmonary diseases abiding by the goldilocks principle. Am J Respir Cell Mol Biol 64:536–546. https://doi.org/10.1165/rcmb.2020-0470TR
Obayashi Y, Yamadori I, Fujita J, Yoshinouchi T, Ueda N, Takahara J (1997) The role of neutrophils in the pathogenesis of idiopathic pulmonary fibrosis. Chest 112:1338–1343
Baumann M, Pham CTN, Benarafa C (2013) SerpinB1 is critical for neutrophil survival through cell-autonomous inhibition of cathepsin G. Blood 121:3900–3907. http://ashpublications.org/blood/article-pdf/121/19/3900/1218553/3900.pdf
Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L et al (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinform 2008:420747. https://doi.org/10.1155/2008/420747
Gong D, Farley K, White M, Hartshorn KL, Benarafa C, Remold-O’Donnell E (2011) Critical role of serpinB1 in regulating inflammatory responses in pulmonary influenza infection. J Infect Dis 204:592–600. https://doi.org/10.1093/infdis/jir352
Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR (2008) The role of urokinase in idiopathic pulmonary fibrosis and implication for therapy. Expert Opin Investig Drugs 17:905–916. https://doi.org/10.1517/13543784.17.6.905
Tucker TA, Idell S (2021) The contribution of the urokinase plasminogen activator and the urokinase receptor to pleural and parenchymal lung injury and repair: a narrative review. Int J Mol Sci 22:1437. https://doi.org/10.3390/ijms22031437
Idell S, Cohen AB (1985) Bronchoalveolar lavage in patients with the adult respiratory distress syndrome. Clin Chest Med 6:459–471
Chang LC, Tseng JC, Hua CC, Liu YC, Shieh W Bin, Wu HP (2006) Gene polymorphisms of fibrinolytic enzymes in coal workers’ pneumoconiosis. Arch Environ Occup Health 61:61–66. https://pubmed.ncbi.nlm.nih.gov/17649957/
Acknowledgements
We are indebted to Instituto de Saúde Pública da Universidade do Porto (ISPUP) for providing samples from the EPIPorto cohort to be used as controls; we thank the cohort management and all participants. We acknowledge the contribution of Ana Isabel Loureiro, MD (Centro Hospitalar de Trás-os-Montes e Alto Douro) and Rui Rolo, MD (Hospital de Braga) who referred some IPF patients to our center. We are grateful to Catarina Teixeira Antunes and Joana Filipa Amorim Reis for assistance in the genotyping.
Funding
This work was supported by the Portuguese Society of Pulmonology (SPP) (Grant - SPP/Bolsa Novartis 2012) and Boehringer-Ingelheim (unrestricted research grant).
Author information
Authors and Affiliations
Contributions
Conceptualization: P.C.M., M.L.S., J.A.M., and A.M.; methodology: P.C.M., M.L.S., A.M., and E.M.; data curation: P.C.M., M.L.S., and B.A.L.; formal analysis: P.C.M., M.L.S., B.A.L., and E.M.; investigation: P.C.M., M.L.S., C.D.V., A.C.F., N.M., H.N-B., and A.M.; validation: P.C.M., M.L.S., and J.H.M.; writing—original draft: P.C.M., M.L.S., and B.A.L.; writing—review and editing: all authors; resources: P.C.M., M.L.S., A.C.F., N.M., and H. N-B; funding acquisition: P.C.M., M.L.S., J.A.M., and A.M.; supervision and project administration: P.C.M., M.L.S., and A.M.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Centro Hospitalar Universitário de São João (CHUSJ) (CE-203–2012).
Consent to participate
Written informed consent was obtained from all individual participants in the study.
Competing interests
Patrícia Caetano Mota has received speaker fees from Boehringer-Ingelheim and research grants from Boehringer-Ingelheim and Novartis; and has participated in research with Boehringer-Ingelheim and Roche, for which her institution has been remunerated.
Hélder Novais-Bastos and António Morais have attended advisory boards for Boehringer-Ingelheim and Roche; have received speaker fees from Boehringer-Ingelheim and Roche; have participated in research with Boehringer-Ingelheim and Roche, for which their institution has been remunerated. Hélder Novais-Bastos has also received a research grant from Boehringer-Ingelheim.
The remaining authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mota, P.C., Soares, M.L., Vasconcelos, C.D. et al. Predictive value of common genetic variants in idiopathic pulmonary fibrosis survival. J Mol Med 100, 1341–1353 (2022). https://doi.org/10.1007/s00109-022-02242-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02242-y