Abstract
RNA methylation is emerging as an important regulator of gene expression. Dysregulation of methyltransferase that is essential for RNA modification contributes to the development and progression of human cancers. Here we show that methyltransferase-like 1 (METTL1) is upregulated in hepatocellular carcinoma (HCC) and exhibits oncogenic activities via PTEN/AKT signaling pathway. High expression of METTL1 is correlated with larger tumor size, higher serum AFP level, tumor vascular invasion, and poor prognosis in two independent cohorts containing 892 patients with HCC. Multivariate analyses suggest METTL1 as an independent factor for unfavorable overall survival. In vitro studies demonstrate that METTL1 overexpression promotes cell proliferation and migration, whereas its knockdown results in opposite phenotypes. Gene set enrichment analysis (GSEA) indicates PTEN pathway is activated in patients with low METTL1 expression. Ectopic expression of PTEN or inhibition of AKT activity significantly attenuates the METTL1-mediated malignant phenotypes. In clinical samples, METTL1 expression is reversely associated with PTEN expression. Combination of low METTL1 expression and high PTEN expression is significantly correlated with overall survival, more so than either METTL1 or PTEN expression alone. Collectively, our data suggest that METTL1 serves as a promising prognostic biomarker and that targeting METTL1/PTEN axis may provide therapeutic potential in HCC intervention.
Key messages
-
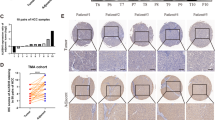
METTL1 is upregulated in HCC and correlated with poor outcomes.
-
METTL1 promotes cell proliferation and migration in HCC.
-
METTL1 exerts oncogenic activities via suppression of PTEN signaling.





Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132
Dhanasekaran R, Nault JC, Roberts LR, Zucman-Rossi J (2018) Genomic medicine and implications for hepatocellular carcinoma prevention and therapy. Gastroenterology. 156:492–509
Deng X, Su R, Weng H, Huang H, Li Z, Chen J (2018) RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res 28:507–517
Dowling DP, Miles ZD, Kohrer C, Maiocco SJ, Elliott SJ, Bandarian V, Drennan CL (2016) Molecular basis of cobalamin-dependent RNA modification. Nucleic Acids Res 44:9965–9976
Zhang Z, Park E, Lin L, Xing Y (2018) A panoramic view of RNA modifications: exploring new frontiers. Genome Biol 19:11
Frye M, Harada BT, Behm M, He C (2018) RNA modifications modulate gene expression during development. Science 361:1346–1349
Bahr A, Hankeln T, Fiedler T, Hegemann J, Schmidt ER (1999) Molecular analysis of METTL1, a novel human methyltransferase-like gene with a high degree of phylogenetic conservation. Genomics 57:424–428
Dewe JM, Whipple JM, Chernyakov I, Jaramillo LN, Phizicky EM (2012) The yeast rapid tRNA decay pathway competes with elongation factor 1A for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA 18:1886–1896
Cartlidge RA, Knebel A, Peggie M, Alexandrov A, Phizicky EM, Cohen P (2005) The tRNA methylase METTL1 is phosphorylated and inactivated by PKB and RSK in vitro and in cells. EMBO J 24:1696–1705
Lin S, Liu Q, Lelyveld VS, Choe J, Szostak JW, Gregory RI (2018) Mettl1/Wdr4-mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol Cell 71:244–255 e245
Boulias K, Greer EL (2019) Put the pedal to the METTL1: adding internal m(7)G increases mRNA translation efficiency and augments miRNA processing. Mol Cell 74:1105–1107
Zhang LS, Liu C, Ma H, Dai Q, Sun HL, Luo G, Zhang Z, Zhang L, Hu L, Dong X, He C (2019) Transcriptome-wide mapping of internal N(7)-methylguanosine methylome in mammalian mRNA. Mol Cell 74:1304–1316 e1308
Shaheen R, Abdel-Salam GM, Guy MP, Alomar R, Abdel-Hamid MS, Afifi HH, Ismail SI, Emam BA, Phizicky EM, Alkuraya FS (2015) Mutation in WDR4 impairs tRNA m(7)G46 methylation and causes a distinct form of microcephalic primordial dwarfism. Genome Biol 16:210
Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM (2006) Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21:87–96
Okamoto M, Fujiwara M, Hori M, Okada K, Yazama F, Konishi H, Xiao Y, Qi G, Shimamoto F, Ota T, Temme A, Tatsuka M (2014) tRNA modifying enzymes, NSUN2 and METTL1, determine sensitivity to 5-fluorouracil in HeLa cells. PLoS Genet 10:e1004639
Pandolfini L, Barbieri I, Bannister AJ, Hendrick A, Andrews B, Webster N, Murat P, Mach P, Brandi R, Robson SC, Migliori V, Alendar A, d’Onofrio M, Balasubramanian S, Kouzarides T (2019) METTL1 promotes let-7 microRNA processing via m7G methylation. Mol Cell 74:1278–1290 e1279
Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A, Wadi L, Meyer M, Wong J, Xu C, Merico D, Bader GD (2019) Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc 14:482–517
Zhang CZ, Chen SL, Wang CH, He YF, Yang X, Xie D, Yun JP (2018) CBX8 exhibits oncogenic activity via AKT/beta-catenin activation in hepatocellular carcinoma. Cancer Res 78:51–63
Yang YF, Zhang MF, Tian QH, Fu J, Yang X, Zhang CZ, Yang H (2018) SPAG5 interacts with CEP55 and exerts oncogenic activities via PI3K/AKT pathway in hepatocellular carcinoma. Mol Cancer 17:117
Fu J, Qiu H, Cai M, Pan Y, Cao Y, Liu L, Yun J, Zhang CZ (2013) Low cyclin F expression in hepatocellular carcinoma associates with poor differentiation and unfavorable prognosis. Cancer Sci 104:508–515
Stoecklin G, Diederichs S (2014) tRNAs: new tricks from old dogs. EMBO J 33:1981–1983
Wikman H, Nymark P, Vayrynen A, Jarmalaite S, Kallioniemi A, Salmenkivi K, Vainio-Siukola K, Husgafvel-Pursiainen K, Knuutila S, Wolf M et al (2005) CDK4 is a probable target gene in a novel amplicon at 12q13.3-q14.1 in lung cancer. Genes Chromosom Cancer 42:193–199
Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM et al (2018) RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology 67:2254–2270
Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, Wang TT, Xu QG, Zhou WP, Sun SH (2017) METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary microRNA processing. Hepatology 65:529–543
Kuijk EW, van Mil A, Brinkhof B, Penning LC, Colenbrander B, Roelen BA (2010) PTEN and TRP53 independently suppress Nanog expression in spermatogonial stem cells. Stem Cells Dev 19:979–988
He M, Zheng B, Zhang Y, Zhang XH, Wang C, Yang Z, Sun Y, Wu XL, Wen JK (2015) KLF4 mediates the link between TGF-beta1-induced gene transcription and H3 acetylation in vascular smooth muscle cells. FASEB J 29:4059–4070
Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, Schulman J, Famulare C, Patel M, Klimek VM, Garrett-Bakelman FE, Melnick A, Carroll M, Mason CE, Jaffrey SR, Kharas MG (2017) The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med 23:1369–1376
Weng YL, Wang X, An R, Cassin J, Vissers C, Liu Y, Liu Y, Xu T, Wang X, Wong SZH, Joseph J, Dore LC, Dong Q, Zheng W, Jin P, Wu H, Shen B, Zhuang X, He C, Liu K, Song H, Ming GL (2018) Epitranscriptomic m(6)a regulation of axon regeneration in the adult mammalian nervous system. Neuron 97:313–325 e316
Funding
The study was supported by the National Natural Science Foundation of China (No. 81960441).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Written informed consent was obtained from all patients involved in this study. The use of tissues in this study has been approved by the Institute Research Medical Ethics Committees of SYSUCC and The First Affiliated Hospital of Nanchang University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
The genomic alteration of METTL1 in malignancies in TCGA dataset. (PDF 31.9 kb)
Supplementary Figure 2
AKT activity is involved in METTL1-mediated phenotypes. A. HepG2 and Huh7 cells with METTL1 overexpression were treated with 1 μM MK2206, a specific inhibitor of AKT, for 24 h. Colony formation and transwell assays were performed. B. HCC cells treated with METTL1 siRNA were incubated with 100 nM insulin, an AKT activator, for 24 h. Cell proliferation and migration were evaluated. *P < 0.05. (JPG 369 kb)
Supplementary Figure 3
The effect of NANOG on METTL1-mediated cell growth and migration. A. Cells were transfected with METTL1 siRNA for 36 h. The expression of METTL1, NANOG and KLF4 were determined by Western blot. B. The effect of NANOG on METTL1-mediated cell proliferation and migration were tested using rescue experiments. All *P < 0.05. (JPG 176 kb)
Rights and permissions
About this article
Cite this article
Tian, QH., Zhang, MF., Zeng, JS. et al. METTL1 overexpression is correlated with poor prognosis and promotes hepatocellular carcinoma via PTEN. J Mol Med 97, 1535–1545 (2019). https://doi.org/10.1007/s00109-019-01830-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01830-9