Abstract
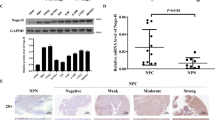
Lymph node metastasis (N classification) is one of the most important prognostic factors of nasopharyngeal carcinoma (NPC), and nerve involvement is associated with the transition of the N category in NPC patients. Although the nervous system has been reported to participate in many types of cancer progression, its functions in NPC progression remains unknown. Through analysis of gene profiling data, we demonstrate an enrichment of genes associated with neuronal development and differentiation in NPC tissues and cell lines. Among these genes, Nogo receptor 3 (NgR3), which was originally identified in the nervous system and plays a role in nerve development and regeneration, was inappropriately overexpressed in NPC cells and tissues. Immunohistochemical analysis demonstrated that the overexpression of NgR3 was correlated with poor prognosis in NPC patients. Overexpression of NgR3 promoted, and knocking down NgR3 inhibited, NPC cell migration and invasion in vitro and metastasis in vivo. The ability of NgR3 to promote cell migration was triggered by the downregulation of E-cadherin and enhanced cytoskeletal rearrangement and cell polarity, which were correlated with the activation of focal adhesion kinase (FAK). Collectively, NgR3 is a novel indicator of poor outcomes in NPC patients and plays an important role in driving the progression of NPC. These results suggest a potential link between the nervous system and NPC progression.
Key messages
-
Genes involved in the neuronal biological process are enriched in nasopharyngeal carcinoma.
-
Overexpression of NgR3 correlates with poor prognosis of nasopharyngeal carcinoma.
-
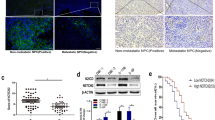
NgR3 promotes NPC cell migration by downregulating E-cadherin.
-
NgR3 promotes NPC cell polarity and enhances the formation of NPC cell pseudopodia by activating FAK/Src pathway.






Similar content being viewed by others
References
Cao SM, Simons MJ, Qian CN (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30(2):114–119
Riaz N, Tam M and Lee N (2014) Nasopharyngeal Carcinoma. Target Volume Delineation for Conformal and Intensity-Modulated Radiation Therapy. 1st edn. ed N Y Lee et al. Springer, Berlin, pp 3-5
Farias TP, Dias FL, Lima RA, Kligerman J, de Sa GM, Barbosa MM et al (2003) Prognostic factors and outcome for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 129(7):794–799
Liu MT, Hsieh CY, Chang TH, Lin JP, Huang CC, Wang AY (2003) Prognostic factors affecting the outcome of nasopharyngeal carcinoma. Jpn J Clin Oncol 33(10):501–508
Yamashita S, Kondo M, Hashimoto S (1985) Squamous cell carcinoma of the nasopharynx. An analysis of failure patterns after radiation therapy. Acta Radiol Oncol 24(4):315–320
Chen YP, Tang LL, Zhang WN, Mao YP, Chen L, Sun Y, Liu LZ, Li WF, Liu X, Zhou GQ et al (2015) Prognostic value and grading of MRI-based T category in patients with nasopharyngeal carcinoma without lymph node metastasis undergoing intensity-modulated radiation therapy. Medicine 94(43):e1624
Lan M, Huang Y, Chen CY, Han F, Wu SX, Tian L, Zheng L, Lu TX (2015) Prognostic value of cervical nodal necrosis in nasopharyngeal carcinoma: analysis of 1800 patients with positive cervical nodal metastasis at MR imaging. Radiology 276(2):536–544
Lee A, Poon YF, Foo W, Law SC, Cheung FK, Chan DK (1992) Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys 23(2):261–270
Heng DM, Wee J, Fong KW, Lian LG, Sethi VK, Chua ET et al (1999) Prognostic factors in 677 patients in Singapore with nondisseminated nasopharyngeal carcinoma. Cancer 86(10):1912–1920
Lee AW, Foo W, Law SC, Poon YF, O SK, Tung SY et al (1999) Staging of nasopharyngeal carcinoma: from Ho's to the new UICC system. Int J Cancer 84(2):179–187
Liu L, Liang S, Li L, Mao Y, Tang L, Tian L, Liao X, Cui C, Lin A, Ma J (2009) Prognostic impact of magnetic resonance imaging-detected cranial nerve involvement in nasopharyngeal carcinoma. Cancer 115(9):1995–2003
Li JX, Lu TX, Huang Y, Han F, Chen CY, Xiao WW (2010) Clinical features of 337 patients with recurrent nasopharyngeal carcinoma. Chin J Cancer 29(1):82–86
Au JS, Law CK, Foo W, Lau WH (2003) In-depth evaluation of the AJCC/UICC 1997 staging system of nasopharyngeal carcinoma: prognostic homogeneity and proposed refinements. Int J Radiat Oncol Biol Phys 56(2):413–426
Gruner A, Grabenbauer GG, Rodel C, Weidenbecher M, Martus P, Iro H et al (1999) Nasopharyngeal carcinoma: only irradiation or simultaneous radiochemotherapy? Strahlenther Onkol 175(12):591–596 Nasopharynxkarzinom: nur Bestrahlung oder simultane Radiochemotherapie? ger
Huang W, Mo H, Deng M, Mai H, Qi B, Li J et al (2009) Relationship between cranial nerve involvement in nasopharyngeal carcinoma and the prognosis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 23(21):964–967
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341(6142):1236361
Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D (2001) In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49(3):213–223
Schuller HM, Al-Wadei HA, Ullah MF, Plummer HK 3rd (2012) Regulation of pancreatic cancer by neuropsychological stress responses: a novel target for intervention. Carcinogenesis 33(1):191–196
Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12(8):939–944
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K (2011) Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol 29(19):2635–2644
Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F et al (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 29(19):2645–2652
Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, Lesinski GB, Jackson R, Glaser R (2011) Beta-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomark Prev 20(10):2273–2279
Grytli HH, Fagerland MW, Fossa SD, Tasken KA (2014) Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 65(3):635–641
Descamps S, Pawlowski V, Revillion F, Hornez L, Hebbar M, Boilly B et al (2001) Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res 61(11):4337–4340
Luchino J, Hocine M, Amoureux MC, Gibert B, Bernet A, Royet A, Treilleux I, Lécine P, Borg JP, Mehlen P et al (2013) Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell 24(5):673–685
Katoh M, Katoh M (2006) Comparative integromics on Eph family. Int J Oncol 28(5):1243–1247
Zeng L, Tian YM, Huang Y, Sun XM, Wang FH, Deng XW, Han F, Lu TX (2014) Retrospective analysis of 234 nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis: therapeutic approaches and prognostic factors. PLoS One 9(9):e108070
Suarez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A (2010) Current treatment options for recurrent nasopharyngeal cancer. Europ Arch Otorhinolaryngol 267(12):1811–1824
Oliveros JC (2007) Venny. An interactive tool for comparing lists with Venn's diagrams. Available from: http://bioinfogp.cnb.csic.es/tools/venny/index.html
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1):44–57
da Huang W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37(1):1–13
Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M et al (2003) TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34(2):374–378
Dimri GP, Itahana K, Acosta M, Campisi J (2000) Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol Cell Biol 20(1):273–285
Song LB, Zeng MS, Liao WT, Zhang L, Mo HY, Liu WL, Shao JY, Wu QL, Li MZ, Xia YF et al (2006) Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res 66(12):6225–6232
Li L, Lee KM, Han W, Choi JY, Lee JY, Kang GH, Park SK, Noh DY, Yoo KY, Kang D (2010) Estrogen and progesterone receptor status affect genome-wide DNA methylation profile in breast cancer. Hum Mol Genet 19(21):4273–4277
Wang J, Scholtens D, Holko M, Ivancic D, Lee O, Hu H, Chatterton RT, Sullivan ME, Hansen N, Bethke K et al (2013) Lipid metabolism genes in contralateral unaffected breast and estrogen receptor status of breast cancer. Cancer Prev Res (Phila) 6(4):321–330
Gabrielli F, Tofanelli S (2012) Molecular and functional evolution of human DHRS2 and DHRS4 duplicated genes. Gene 511(2):461–469
Kisiela M, El-Hawari Y, Martin HJ, Maser E (2011) Bioinformatic and biochemical characterization of DCXR and DHRS2/4 from Caenorhabditis elegans. Chem Biol Interact 191(1–3):75–82
Caroni P, Schwab ME (1988) Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1(1):85–96
Pignot V, Hein AE, Barske C, Wiessner C, Walmsley AR, Kaupmann K, Mayeur H, Sommer B, Mir AK, Frentzel S (2003) Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. J Neurochem 85(3):717–728
Schwab ME (2010) Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci 11(12):799–811
Sizhong Z, Xiukung G, Yi Z (1983) Cytogenetic studies on an epithelial cell line derived from poorly differentiated nasopharyngeal carcinoma. Int J Cancer 31(5):587–590
Li J, Fan Y, Chen J, Yao KT, Huang ZX (2010) Microarray analysis of differentially expressed genes between nasopharyngeal carcinoma cell lines 5-8F and 6-10B. Cancer Genet Cytogenet 196(1):23–30
Glaser R, Zhang HY, Yao KT, Zhu HC, Wang FX, Li GY, Wen DS, Li YP (1989) Two epithelial tumor cell lines (HNE-1 and HONE-1) latently infected with Epstein-Barr virus that were derived from nasopharyngeal carcinomas. Proc Natl Acad Sci U S A 86(23):9524–9528
Dong JQ, Li MZ, Liu ZG, Zhong Q, Xiong D, Xu LH, Du Y, Xia YF, Zeng MS (2012) Establishment and characterization of a novel nasopharyngeal carcinoma cell line (SUNE2) from a Cantonese patient. Chin J Cancer 31(1):36–44
Huang DP, Ho JH, Poon YF, Chew EC, Saw D, Lui M et al (1980) Establishment of a cell line (NPC/HK1) from a differentiated squamous carcinoma of the nasopharynx. Int J Cancer 26(2):127–132
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292
Singh A, Settleman J (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29(34):4741–4751
Christofori G (2006) New signals from the invasive front. Nature 441(7092):444–450
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9(4):265–273
Yilmaz M, Christofori G (2009) EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev 28(1–2):15–33
Schmalhofer O, Brabletz S, Brabletz T (2009) E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev 28(1–2):151–166
Canel M, Serrels A, Frame MC, Brunton VG (2013) E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci 126(Pt 2):393–401
Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM (2010) Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol 26(1):315–333
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003) Cell migration: integrating signals from front to back. Science 302(5651):1704–1709
Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell 84(3):359–369
Yamazaki D, Kurisu S, Takenawa T (2005) Regulation of cancer cell motility through actin reorganization. Cancer Sci 96(7):379–386
Spiering D, Hodgson L (2011) Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhes Migr 5(2):170–180
Le Clainche C, Carlier MF (2008) Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev 88(2):489–513
Sturge J, Wienke D, Isacke CM (2006) Endosomes generate localized Rho-ROCK-MLC2-based contractile signals via Endo180 to promote adhesion disassembly. J Cell Biol 175(2):337–347
Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G (2009) VE-cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol 19(8):668–674
Kupfer A, Louvard D, Singer SJ (1982) Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci U S A 79(8):2603–2607
Bershadsky AD, Futerman AH (1994) Disruption of the Golgi apparatus by brefeldin A blocks cell polarization and inhibits directed cell migration. Proc Natl Acad Sci U S A 91(12):5686–5689
Webb DJ, Parsons JT, Horwitz AF (2002) Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat Cell Biol 4(4):E97–100
Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF (2004) FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6(2):154–161
Horton ER, Humphries JD, Stutchbury B, Jacquemet G, Ballestrem C, Barry ST, Humphries MJ (2016) Modulation of FAK and Src adhesion signaling occurs independently of adhesion complex composition. J Cell Biol 212(3):349–364
Wu JC, Chen YC, Kuo CT, Wenshin Yu H, Chen YQ, Chiou A et al (2015) Focal adhesion kinase-dependent focal adhesion recruitment of SH2 domains directs SRC into focal adhesions to regulate cell adhesion and migration. Sci Rep 5:18476
Gonzalez Wusener AE, Gonzalez A, Nakamura F, Arregui CO (2015) PTP1B triggers integrin-mediated repression of myosin activity and modulates cell contractility. Biol Open 5(1):32–44
Semavina M, Saha N, Kolev MV, Goldgur Y, Giger RJ, Himanen JP, Nikolov DB (2011) Crystal structure of the Nogo-receptor-2. Protein Sci 20(4):684–689
Schweigreiter R, Walmsley AR, Niederost B, Zimmermann DR, Oertle T, Casademunt E et al (2004) Versican V2 and the central inhibitory domain of Nogo-A inhibit neurite growth via p75NTR/NgR-independent pathways that converge at RhoA. Mol Cell Neurosci 27(2):163–174
Palandri A, Salvador VR, Wojnacki J, Vivinetto AL, Schnaar RL, Lopez PH (2015) Myelin-associated glycoprotein modulates apoptosis of motoneurons during early postnatal development via NgR/p75(NTR) receptor-mediated activation of RhoA signaling pathways. Cell Death Dis 6(9):e1876
Yan J, Zhou X, Guo JJ, Mao L, Wang YJ, Sun J, Sun LX, Zhang LY, Zhou XF, Liao H (2012) Nogo-66 inhibits adhesion and migration of microglia via GTPase Rho pathway in vitro. J Neurochem 120(5):721–731
Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE (2002) Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci 22(23):10368–10376
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study was approved by the Institute Research Ethics Committee at the Sun Yat-Sen Memorial Hospital. Written informed consent was obtained from each patient.
Conflict of interest
The authors declare no potential competing interest.
Electronic supplementary material
Supplementary table S1
(XLSX 19 kb)
Supplementary table S2
(XLSX 12 kb)
Supplementary table S3
(XLSX 10 kb)
Supplementary Figure S1
RTN4RL1 expression in NPC cell lines. (a) RTN4RL1 mRNA expression level in nasopharyngeal epithelial cell lines, data set GSE15903. (GIF 48 kb)
Supplementary Figure S2
The role of NgR3 in proliferation and clonal formation of NPC cells. (a and b) MTT assay of NgR3 stably overexpressed CNE1 and S26 cell lines cultured in 5% FBS RPMI 1640. (c) MTT assay of NgR3 stably knockdown CNE2 cell line, cultured in 5% FBS 1640. (d and f) Clonal formation assay of CNE1 and S26 cell lines stably overexpression of NgR3. (e and g) Statistical results of D and F. All experiments were performed in biological triplicates with three technical replicates and values represented the mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significant. (GIF 266 kb)
Supplementary Figure S3
Expression level of classical ligands and co-receptors of NgR3 are not changed in nasopharyngeal tissues and cell lines. (a) Expression of NogoA in nasopharyngeal epithelial cell lines. (b) Expression of MAG in nasopharyngeal epithelial cell lines. (c) Expression of OMgP in nasopharyngeal epithelial cell lines. (d) Expression of TROY in nasopharyngeal epithelial cell lines. (e) Hot-map of NgR3 ligands and co-receptors expression in data set GSE53819. (f) Fold change of NgR3’s ligands and co-receptors. (g-k) qPCR to detect expression of RTN4RL1’s ligands and co-receptors in non-cancerous nasopharyngeal tissues and NPC tissues. All experiments were performed in biological triplicates with three technical replicates and values represented the mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significant. (GIF 262 kb)
Rights and permissions
About this article
Cite this article
He, JY., Han, P., Zhang, Y. et al. Overexpression of Nogo receptor 3 (NgR3) correlates with poor prognosis and contributes to the migration of epithelial cells of nasopharyngeal carcinoma patients. J Mol Med 96, 265–279 (2018). https://doi.org/10.1007/s00109-017-1618-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1618-1