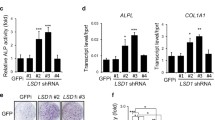
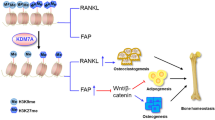
Abstract
Excess glucocorticoid administration impairs osteogenic activities, which raises the risk of osteoporotic disorders. Epigenetic methylation of DNA and histone regulates the lineage commitment of progenitor cells. This study was undertaken to delineate the actions of histone lysine demethylase 6a (UTX) with regard to the glucocorticoid impediment of osteogenic differentiation. Osteogenic progenitor cells responded to supraphysiological glucocorticoid by elevating CpG dinucleotide methylation proximal to transcription start sites within Runx2 and osterix promoters and Wnt inhibitor Dickkopf-1 (Dkk1) expression concomitant with low UTX expression. 5′-Aza-deoxycystidine demethylation of Runx2 and osterix promoters abolished the glucocorticoid inhibition of mineralized matrix accumulation. Gain of UTX function attenuated the glucocorticoid-induced loss of osteogenic differentiation, whereas UTX silencing escalated adipogenic gene expression and adipocyte formation. UTX sustained osteogenic gene transcription through maintaining its occupancy to Runx2 and osterix promoters. It also mitigated the trimethylation of histone 3 at lysine 27 (H3K27me3), which reduced H3K27me3 enrichment to Dkk1 promoter and thereby lowered Dkk1 transcription. Modulation of β-catenin and Dkk1 actions restored UTX signaling in glucocorticoid-stressed cells. In vivo, UTX inhibition by exogenous methylprednisolone and GSK-J4 administration, an effect that disturbed H3K27me3, β-catenin, Dkk1, Runx2, and osterix levels, exacerbated trabecular microarchitecture loss and marrow adiposity. Taken together, glucocorticoid reduction of UTX function hindered osteogenic differentiation. Epigenetic hypomethylation of osteogenic transcription factor promoters and H3K27 contributed to the UXT alleviation of Dkk1 transcription and osteogenesis in glucocorticoid-stressed osteogenic progenitor cells. Control of UTX action has an epigenetic perspective of curtailing glucocorticoid impairment of osteogenic differentiation and bone mass.
Key messages
-
UTX attenuates glucocorticoid deregulation of osteogenesis and adipogenesis.
-
UTX reduces Runx2 promoter methylation and H3K27me3 enrichment in the Dkk1 promoter.
-
β-catenin and Dkk1 modulate the glucocorticoid inhibition of UTX signaling.
-
UTX inhibition exacerbates bone mass, trabecular microstructure and fatty marrow.
-
UTX signaling is indispensable in fending off glucocorticoid-impaired osteogenesis.








Similar content being viewed by others
References
Leib ES, Winzenrieth R (2016) Bone status in glucocorticoid-treated men and women. Osteoporosis Int 27:39–48
Rizzoli R, Biver E (2015) Glucocorticoid-induced osteoporosis: who to treat with what agent? Nat Rev Rheumatol 11:98–109
Li H, Li T, Fan J, Li T, Fan L, Wang S, Weng X, Han Q, Zhao RC (2015) miR-216 rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cb1-mediated PI3K/AKT pathway. Cell Death Differ 22:1935–1945
Zhang Y, Ma C, Liu X, Wu Z, Yan P, Ma N, Fan Q, Zhao Q (2015) Epigenetic landscape in PPARγ2 in the enhancement of adipogenesis of mouse osteoporotic bone marrow stromal cells. Biochim Biophys Acta 1852:2504–2516
Tamura Y, Kawao N, Yano M, Okada K, Okumoto K, Chiba Y, Matsuo O, Kaji H (2015) Role of plasminogen activator inhibitor-1 in glucocorticoid-induced diabetes and osteopenia in mice. Diabetes 64:2194–2206
Ko JY, Chuang PC, Su WH, Ke HC, Chen YS, Sun YC, Wang FS (2015) MicroRNA-29a mitigates glucocorticoid induction of bone loss and fatty marrow by rescuing Runx2 acetylation. Bone 81:80–88
Matsumura Y, Nakaki R, Inagaki T, Yoshida A, Kano Y, Kimura H, Tanaka T, Tsutsumi S, Nakao M, Doi T et al (2015) H3K4/H3K9me3 bivalent chromatin domains targeted by lineage-specific DNA methylation pauses adipocyte differentiation. Mol Cell 60:584–596
Pathania R, Ramachandran S, Elangovan S, Padia R, Yang P, Cignhu S, Veeranan-Karmegam R, Arjunan P, Gnana-Prakasam JP, Sadanand F et al (2015) DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat Commun 6:6910
Hussein SM, Puri MC, Tonge PD, Benevento M, Corso AJ, Clancy JL, Mosbergen R, Li M, Lee DS, Cloonan N et al (2014) Genome-wide characterization of the routes to pluripotency. Nature 516:198–206
Berdasco M, Melguizo C, Prados J, Gómez A, Alaminos M, Pujana MA, Lopez M, Setien F, Ortiz R, Zafra I et al (2012) DNA methylation plasticity of human adipose-derived stem cells in lineage commitment. Am J Pathol 181:2079–2093
Delgado-Calle J, Saňudo C, Sánchez-Verde L, García-Renedo RJ, Arozamena J, Riancho JA (2011) Epigenetic regulation of alkaline phosphatase in human cells of the osteoblastic lineage. Bone 49:830–838
Yan X, Ehnert S, Culmes M, Bachmann A, Seeliger C, Schyschka L, Wang Z, Rahmanian-Schwarz A, Stöckle U, De Sousa PA et al (2014) 5-Azacytidine improves the osteogenic differentiation potential of aged human adipose-derived mesenchymal stem cells by DNA demethylation. PLoS One 9:e90846
Cho YD, Yoon WJ, Kim WJ, Woo KM, BaeK JH, Lee G, Ku Y, van Wijnen AJ, Ryoo HM (2014) Epigenetic modifications and canonical wingless/int-1 class (WNT) signaling enable trans-differentiation of nonosteogenic cells into osteoblasts. J Biol Chem 289:20120–20128
Kim HJ, Park JW, Lee KH, Yoon H, Shin DH, Ju UI, Seok SH, Lim SH, Lee ZH, Kim HH et al (2014) Plant homeodomain finger protein 2 promotes bone formation by methylating and activating Runx2 for osteoblast differentiation. Cell Res 24:1231–1249
Dimitrova E, Turberfield AH, Klose RJ (2015) Histone demethylase in chromatin and beyond. EMBO Rep 16:1620–1639
Dudakovic A, Camilleri ET, Xu F, Riester SM, McGee-Lawrence ME, Bradley EW, Paradise CR, Lewallen EA, Thaler R, Devle DR et al (2015) Epigenetic control of skeletal development by the histone methyltransferase Ezh2. J Biol Chem 290:2760–27617
Sinha KM, Yasuda H, Zhou X, deCrombrugghe B (2014) Osterix and NO66 histone demethylase control the chromatin of osterix target genes during osteoblast differentiation. J Bone Miner Res 29:855–865
Ge W, Liu Y, Chen T, Zhang X, Lv L, Jin C, Jiang Y, Shi L, Zhou Y (2014) The epigenetic promotion of osteogenic differentiation of human adipose-derived stem cells by the genetic and chemical blockade of histone demethylase LSD1. Biomaterials 35:6015–6025
Manna A, Kim JK, Baugé CM, Zhao Y, Shetty J, Vacchio MS, Castro E, Tran B, Tessarollo L, Bosselut R (2015) Histone H3 lysine 27 demethylase Jmjd3 and Utx are required for T-cell differentiation. Nat Commun 6:8152
Chakroum I, Yang D, Girgis J, Gunasekharan A, Phenix H, Kærn M, Blais A (2015) Genome-wide association between Six4, MyoD, and the histone demethylase Utx during myogenesis. FASEB J 29:4738–4755
Thieme S, Gyarfas T, Richter C, Ozhan G, Fu J, Alexopoulou D, Muders MH, Michalk I, Jakob C, Dahl A et al (2013) The histone demethylase UTX regulates stem cell migration and hematopoiesis. Blood 121:2462–2473
Ntziachristos P, Tsirigos A, Welstead GG, Trimacrchi T, Bakogianni S, Xu L, Loizou E, Holmfeldt L, Strikoudis A, King B et al (2014) Contrasting roles of histone 3 jyinse 27 demthylase in acute lymphoblastic leukaemia. Nature 514:513–517
Hemming S, Cakouros D, Isenmann S, Cooper L, Menicanin D, Zanettino A, Gronthos S (2014) EZH2 and UTX act as an epigenetic switch to regulate mesenchymal stem cell lineage specification. Stem Cells 32:802–815
Ye L, Fan Z, Yu B, Chang J, Al Hezimi K, Zhou X, Park NH, Wang CY (2012) Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell 11:50–61
Wang FS, Lian WS, Weng WT, Sun YC, Ke HJ, Chen YS, Ko JY (2016) Neuropeptide Y mediates glucocorticoid-induced osteoporosis and marrow adiposity in mice. Osteoporosis Int 27:2777–2789
Krueger F, Kreck B, Franke A, Andrew SR (2012) DNA methylation analysis using short bisulfite sequencing data. Nat Methods 9:145–151
Jiang W, Wang J, Zhang Y (2013) Histone H3K27me3 demethylases UTX and KDM6B modulate definitive endoderm differentiation from human ECSc by regulating Wnt signaling pathways. Cell Res 23:122–130
Lin NY, Chen CW, Kagwiria R, Liang R, Beyer C, Distler A, Luther J, Engelke K, Schette G, Distler JH (2016) Inactivation of autophagy ameliorates glucocorticoid-induced and ovariectomy-induced bone loss. Ann Rheum Dis 75:1203–1210
Kumar Y, Kapoor I, Khan K, Thacker G, Khan MP, Shukla N, Kanaujiya JK, Snayal S, Chattopadhyay N, Trivedi AK (2015) E3 ubiquitin ligase Fbw7 negatively regulates osteoblast differentiation by targeting Runx2 for degradation. J Biol Chem 290:30975–30987
Zhao QH, Wang SG, Liu SX, Li JP, Zhang YX, Sun ZY, Fan QM, Tian JW (2013) PPARγ forms a bridge between DNA methylation and histone acetylation at the C/EBPα gene promoter to regulate the balance between osteogenesis and adipogenesis of bone marrow stromal cells. FEBS J 280:5801–5814
Guo L, Xu K, Qi J, Zhang L, Wang L, Liang L, Qian N, Zhou H, Wei L, Deng L (2013) MicroRNA-17-92a upregulation by estrogen leads to Bim targeting and inhibition of osteoblast apoptosis. J Cell Sci 126:978–988
Seo E, Basu-Roy U, Gunaratne PH, Coarfa C, Lim DS, Basilico C, Mansukhani A (2013) SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep 3:2075–2087
Niziolek PJ, MacDonald BT, Kedlaya R, Zhang M, Bellido T, He X, Warman ML, Robling AG (2015) High bone mass-causing mutant LRP5 receptors are resistant to endogenous inhibitors in vivo. J Bone Miner Res 30:1822–1830
Yuasa M, Yamada T, Taniyama T, Masaoka T, Xuetao W, Yoshii T, Horie M, Yasuda H, Uemura T, Okawa A et al (2015) Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLoS One 10:e0116462
Langenbach F, Handschel J (2013) Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther 5:117
Kang H, Chen H, Huang P, Qi J, Qian N, Deng L, Guo L (2016) Glucocorticoids impair bone formation of bone marrow stromal stem cells by reciprocally regulating microRNA-34a-5p. Osteoporosis Int 27:1493–1505
Hålelien AM, Bryne JC, Harstad KG, Lorenz S, Paulsen L, Sun J, Mikklebost O, Meza-Zepeda LA (2014) The regulatory landscape of osteogenic differentiation. Stem Cells 32:2780–2793
Cho YD, Bae HS, Lee DS, Yoon WJ, Woo KM, Baek JH, Lee G, Park JC, Ku Y, Ryoo HM (2016) Epigenetic priming confers direct cell trans-differentiation from adipocyte to osteoblast in a transgene-free state. J Cell Physiol 231:1484–1494
Azechi T, Sato F, Sudo R, Wachi H (2014) 5-Aza-2′-deoxycytidine, a DNA methyltransferase inhibitor, facilitates the inorganic phosphorous-induced mineralized of vascular smooth muscle cells. J Atheroscler Thromb 21:463–476
Yang D, Okamura H, Teramachi J, Haneji T (2015) Histone demethylase Utx regulates differentiation and mineralization in osteoblasts. J Cell Biochem 116:2628–2636
Rojas A, Aguilar R, Henriquez B, Lian JB, Stein JL, Stein GS, van Wijnen AJ, van Zundert B, Allende ML, Montecino M (2015) Epigenetic control of the bone-master Runx2 gene during osteoblast-lineage commitment by the histone demethyase JARID1B/KDM5B. J Biol Chem 290:28329–28342
Li J, Zhang N, Huang X, Xu J, Fernandes JC, Dai K, Zhang X (2013) Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocyte by C/EBPalpha promoter methylation. Cell Death Dis 4:e832
Ko JY, Wu RW, Kuo SJ, Chen MW, Yeh DW, Ke HCWSL, Wang FS (2012) Cannabinoid receptor 1 mediates glucocorticoid-induced bone loss in rats by perturbing bone mineral acquisition and marrow adipogenesis. Arthritis Rheum 64:1204–1214
Zhang YX, Sun HL, Liang H, Li K, Fan QM, Zhao QH (2015) Dynamic and distinct histone modification of osteogenic genes during osteogenic differentiation. J Biochem 158:445–457
Cai D, Wang J, Jia Y, Liu H, Yuan M, Dong H, Zhao R (2016) Gestational dietary betaine supplementation suppresses hepatic expression of lipogenic genes in neonatal piglets through epigenetic and glucocorticoid receptor-dependent mechanism. Biochem Biophys Acta 186:41–50
Wei Y, Chen YH, Li LY, Lang J, Yeh SP, Shi B, Yang CC, Yang JY, Lin CY, Lai CC et al (2011) CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol 13:87–94
Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS et al (2014) Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell 15:66–78
Acknowledgements
This work was supported in part by grants NHRI-EX105-10436SI from the National Health Research Institute; MOST103-2314-B-182A-053 from the Ministry of Science & Technology; and CLRPG8B0042, CMRPG8B0873, CMRPG8E1321-3, and CMRPG8E0651-3 from Chang Gung Memorial Hospital, Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure statement
The authors have nothing to disclose related to the study.
Research grant support
Grants NHRI-EX105-10436SI from the National Health Research Institute; MOST103-2314-B-182A-053 from the Ministry of Science & Technology; and CLRPG8B0042, CMRPG8B0873, CMRPG8E1321-3, and CMRPG8E651-3 from Chang Gung Memorial Hospital, Taiwan
Additional information
F.-S.W., W.-S.L., and M.S. Lee equally contributed to this study.
Electronic supplementary material
ESM 1
(PDF 155 kb)
Rights and permissions
About this article
Cite this article
Wang, FS., Lian, WS., Lee, M.S. et al. Histone demethylase UTX counteracts glucocorticoid deregulation of osteogenesis by modulating histone-dependent and -independent pathways. J Mol Med 95, 499–512 (2017). https://doi.org/10.1007/s00109-017-1512-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1512-x