Abstract
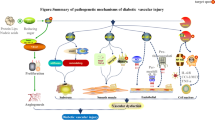
With the increasing incidence of obesity and type 2 diabetes, it is predicted that more than half of Americans will have diabetes or pre-diabetes by 2020. Diabetic patients develop vascular complications at a much faster rate in comparison to non-diabetic individuals, and cardiovascular risk is increased up to tenfold. With the increasing incidence of diabetes across the world, the development of vascular complications will become an increasing medical burden. Diabetic vascular complications affect the micro- and macro-vasculature leading to kidney disease often requiring dialysis and transplantation or cardiovascular disease increasing the risk for myocardial infarction, stroke and amputations as well as leading to premature mortality. It has been suggested that many complex pathways contribute to the pathobiology of diabetic complications including hyperglycaemia itself, the production of advanced glycation end products (AGEs) and interaction with the receptors for AGEs such as the receptor for advanced glycation end products (RAGE), as well as the activation of vasoactive systems such as the renin-angiotensin aldosterone system (RAAS) and the endothelin system. More recently, it has been hypothesised that reactive oxygen species derived from NAD(P)H oxidases (Nox) may represent a common downstream mediator of vascular injury in diabetes. Current standard treatment of care includes the optimization of blood glucose and blood pressure usually including inhibitors of the renin-angiotensin system. Although these interventions are able to delay progression, they fail to prevent the development of complications. Thus, there is an urgent medical need to identify novel targets in diabetic vascular complications which may include the blockade of Nox-derived ROS formation, as well as blockade of AGE formation and inhibitors of RAGE activation. These strategies may provide superior protection against the deleterious effects of diabetes on the vasculature.

Similar content being viewed by others
References
Shaw JE, Sicree RA, Zimmet PZ (2009) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice.
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA et al (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378:31–40
Onkamo P, Väänänen S, Karvonen M, Tuomilehto J (1999) Worldwide increase in incidence of type I diabetes—the analysis of the data on published incidence trends. Diabetologia 42:1395–1403
Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K (2001) Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens 14:475–486
Rahman S, Rahman T, Ismail AA, Rashid AR (2007) Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes, Obesity & Metabolism 9:767–780
Bryden KS, Dunger DB, Mayou RA, Peveler RC, Neil HAW (2003) Poor prognosis of young adults with type 1 diabetes: a longitudinal study. Diabetes Care 26:1052–1057
Diabetes-Australia (2011) Diabetes in Australia; Diabetes Globally.
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Creager MA, Luscher TF, Cosentino F, Beckman JA (2003) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 108:1527–1532
Hurst RT, Lee RW (2003) Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: mechanisms and management. Ann Intern Med 139:824–834
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376:124–136
Durham JT, Herman IM (2011) Microvascular modifications in diabetic retinopathy. Current Diabetes Reports 11:253–264
Najafian B, Alpers CE, Fogo AB (2011) Pathology of human diabetic nephropathy. Contrib Nephrol 170:36–47
Valk EJ, Bruijn JA, Bajema IM (2011) Diabetic nephropathy in humans: pathologic diversity. Curr Opin Nephrol Hypertens 20:285–289
Candido R, Jandeleit-Dahm KA, Cao Z, Nesteroff SP, Burns WC, Twigg SM, Dilley RJ, Cooper ME, Allen TJ (2002) Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation 106:246–253
Watson AM, Olukman M, Koulis C, Tu Y, Samijono D, Yuen D, Lee C, Behm DJ, Cooper ME, Jandeleit-Dahm KA, et al. (2013) Urotensin II receptor antagonism confers vasoprotective effects in diabetes associated atherosclerosis: studies in humans and in a mouse model of diabetes. Diabetologia.
Ago T, Kuroda J, Kamouchi M, Sadoshima J, Kitazono T (2011) Pathophysiological roles of NADPH oxidase/Nox family proteins in the vascular system review and perspective. Circ J 75:1791–1800
Griendling KK, FitzGerald GA (2003) Oxidative stress and cardiovascular injury part I: basic mechanisms and in vivo monitoring of ROS. Circulation 108:1912–1916
Lassègue B, Griendling KK (2010) NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30:653–661
Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL et al (2013) NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127:1888–1902
Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske B, Kutner N et al (2013) US Renal Data System 2012 annual data report. Am J Kidney Dis 61(A7):e1–e476
Van Buren PN, Toto R (2013) Current update in the management of diabetic nephropathy. Current Diabetes Reviews 9:62–77
Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A (2013) Diabetic nephropathy: is it time yet for routine kidney biopsy? World Journal of Diabetes 4:245–255
Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ (2005) Projecting the number of patients with end-stage renal disease in the United States to the year 2015. Journal of the American Society of Nephrology : JASN 16:3736–3741
Mogensen CE, Christensen CK, Vittinghus E (1983) The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32(Suppl 2):64–78
Rasch R, Norgaard JO (1983) Renal enlargement: comparative autoradiographic studies of 3H-thymidine uptake in diabetic and uninephrectomized rats. Diabetologia 25:280–287
UK Prospective Diabetes Study (UKPDS) (1993) X. Urinary albumin excretion over 3 years in diet-treated type 2, (non-insulin-dependent) diabetic patients, and association with hypertension, hyperglycaemia and hypertriglyceridaemia. Diabetologia 36:1021–1029
O'Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE (1996) Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation 93:672–682
Cooper ME, Jandeleit-Dahm K, Thomas MC (2005) Targets to retard the progression of diabetic nephropathy. Kidney Int 68:1439–1445
Lim AK, Tesch GH (2012) Inflammation in diabetic nephropathy. Mediators Inflamm 2012:146154
Tesch GH, Nikolic-Paterson DJ (2006) Recent insights into experimental mouse models of diabetic nephropathy. Nephron Experimental Nephrology 104:e57–e62
Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J (2005) Tubulointerstitial damage and progression of renal failure. Kidney International Supplement: S82-86.
Nangaku M (2006) Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. Journal of the American Society of Nephrology : JASN 17:17–25
Vallon V (2011) The proximal tubule in the pathophysiology of the diabetic kidney. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 300:R1009–R1022
Gilbert RE, Cooper ME (1999) The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 56:1627–1637
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The New England Journal of Medicine 329:1456–1462
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S et al (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. The New England Journal of Medicine 345:861–869
Groop PH, Thomas MC, Moran JL, Waden J, Thorn LM, Makinen VP, Rosengard-Barlund M, Saraheimo M, Hietala K, Heikkila O et al (2009) The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58:1651–1658
AIHW, Tong B, Stevenson C (2007) Comorbidity of cardiovascular disease, diabetes and chronic kidney disease in Australia. AIHW, Canberra
Bloomgarden ZT (2008) Cardiovascular disease in diabetes. Diabetes Care 31:1260–1266
Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC (2003) Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46:760–765
Kanter JE, Averill MM, Leboeuf RC, Bornfeldt KE (2008) Diabetes-accelerated atherosclerosis and inflammation. Circ Res 103:e116–e117
Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, Luo H, van Breemen C (2005) Compromised arterial function in human type 2 diabetic patients. Diabetes 54:2415–2423
Glass CK, Witztum JL (2001) Atherosclerosis. The road ahead. Cell 104:503–516
Soro-Paavonen A, Watson AMD, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI et al (2008) Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 57:2461–2469
Di Marco E, Gray SP, Chew P, Koulis C, Ziegler A, Szyndralewiez C, Touyz RM, Schmidt HH, Cooper ME, Slattery R, Jandeleit-Dahm KA (2013) Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe mice. Diabetologia.
Blasi C (2008) The autoimmune origin of atherosclerosis. Atherosclerosis: In press
Bobryshev YV (2006) Monocyte recruitment and foam cell formation in atherosclerosis. Micron 37:208–222
Burrell LM, Johnston CI, Tikellis C, Cooper ME (2004) ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab 15:166–169
de Gasparo M, Husain A, Alexander W, Catt KJ, Chiu AT, Drew M, Goodfriend T, Harding JW, Inagami T, Timmermans PB (1995) Proposed update of angiotensin receptor nomenclature. Hypertension 25:924–927
Bonnet F, Candido R, Carey RM, Casley D, Russo LM, Osicka TM, Cooper ME, Cao Z (2002) Renal expression of angiotensin receptors in long-term diabetes and the effects of angiotensin type 1 receptor blockade. J Hypertens 20:1615–1624
Candido R, Allen TJ, Lassila M, Cao Z, Thallas V, Cooper ME, Jandeleit-Dahm KA (2004) Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation 109:1536–1542
Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A (2009) Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. The Journal of Clinical Endocrinology and Metabolism 94:2157–2163
Wichi RB, Farah V, Chen Y, Irigoyen MC, Morris M (2007) Deficiency in angiotensin AT1a receptors prevents diabetes-induced hypertension. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 292:R1184–R1189
UK Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Bmj 317:703–713
Sadoshima J (2000) Cytokine actions of angiotensin II. Circ Res 86:1187–1189
Pinaud F, Bocquet A, Dumont O, Retailleau K, Baufreton C, Andriantsitohaina R, Loufrani L, Henrion D (2007) Paradoxical role of angiotensin II type 2 receptors in resistance arteries of old rats. Hypertension 50:96–102
Sourris KC, Morley AL, Koitka A, Samuel P, Coughlan MT, Penfold SA, Thomas MC, Bierhaus A, Nawroth PP, Yamamoto H et al (2010) Receptor for AGEs (RAGE) blockade may exert its renoprotective effects in patients with diabetic nephropathy via induction of the angiotensin II type 2 (AT2) receptor. Diabetologia 53:2442–2451
Esteban V, Lorenzo O, Ruperez M, Suzuki Y, Mezzano S, Blanco J, Kretzler M, Sugaya T, Egido J, Ruiz-Ortega M (2004) Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. Journal of the American Society of Nephrology : JASN 15:1514–1529
Harrison-Bernard LM, Imig JD, Carmines PK (2002) Renal AT1 receptor protein expression during the early stage of diabetes mellitus. Int J Exp Diabetes Res 3:97–108
Li XC, Zhuo JL (2008) Intracellular ANG II directly induces in vitro transcription of TGF-beta1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. American Journal of Physiology Cell Physiology 294:C1034–C1045
Rizkalla B, Forbes JM, Cooper ME, Cao Z (2003) Increased renal vascular endothelial growth factor and angiopoietins by angiotensin II infusion is mediated by both AT1 and AT2 receptors. Journal of the American Society of Nephrology : JASN 14:3061–3071
Koitka A, Cao Z, Koh P, Watson AMD, Sourris KC, Loufrani L, Soro-Paavonen A, Walther T, Woollard KJ, Jandeleit-Dahm KAM et al (2010) Angiotensin II subtype 2 receptor blockade and deficiency attenuate the development of atherosclerosis in an apolipoprotein E-deficient mouse model of diabetes. Diabetologia 53:584–592
Levy BI (2004) Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin-angiotensin system. Circulation 109:8–13
Brede M, Hadamek K, Meinel L, Wiesmann F, Peters J, Engelhardt S, Simm A, Haase A, Lohse MJ, Hein L (2001) Vascular hypertrophy and increased P70S6 kinase in mice lacking the angiotensin II AT(2) receptor. Circulation 104:2602–2607
Iwai M, Chen R, Li Z, Shiuchi T, Suzuki J, Ide A, Tsuda M, Okumura M, Min LJ, Mogi M et al (2005) Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation 112:1636–1643
Senbonmatsu T, Saito T, Landon EJ, Watanabe O, Price E Jr, Roberts RL, Imboden H, Fitzgerald TG, Gaffney FA, Inagami T (2003) A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. The EMBO Journal 22:6471–6482
Harris RC (2012) Podocyte ACE2 protects against diabetic nephropathy. Kidney Int 82:255–256
Gurley SB, Coffman TM (2008) Angiotensin-converting enzyme 2 gene targeting studies in mice: mixed messages. Exp Physiol 93:538–542
Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW (2007) Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. The American Journal of Pathology 171:438–451
Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D (2007) ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int 72:614–623
Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M et al (2003) Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A 100:8258–8263
Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batlle D, Burns KD (2012) Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82:292–303
Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D et al (1999) N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 274:31740–31749
Fu MX, Wells-Knecht KJ, Blackledge JA, Lyons TJ, Thorpe SR, Baynes JW (1994) Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes 43:676–683
Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP (1998) AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus.I. The AGE concept. Cardiovasc Res 37:586–600
Degenhardt TP, Thorpe SR, Baynes JW (1998) Chemical modification of proteins by methylglyoxal. Cell Mol Biol 44:1139–1145
McRobert EA, Gallicchio M, Jerums G, Cooper ME, Bach LA (2003) The amino-terminal domains of the ezrin, radixin, and moesin (ERM) proteins bind advanced glycation end products, an interaction that may play a role in the development of diabetic complications. J Biol Chem 278:25783–25789
Barlovic DP, Thomas MC, Jandeleit-Dahm K (2010) Cardiovascular disease: what's all the AGE/RAGE about? Cardiovascular & Hematological Disorders Drug Targets 10:7–15
Febbraio M, Guy E, Silverstein RL (2004) Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol 24:2333–2338
Lu C, He JC, Cai W, Liu H, Zhu L, Vlassara H (2004) Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci U S A 101:11767–11772
Sourris KC, Forbes JM (2009) Interactions between advanced glycation end-products (AGE) and their receptors in the development and progression of diabetic nephropathy - are these receptors valid therapeutic targets. Curr Drug Targets 10:42–50
Sundblad V, Croci DO, Rabinovich GA (2011) Regulated expression of galectin-3, a multifunctional glycan-binding protein, in haematopoietic and non-haematopoietic tissues. Histol Histopathol 26:247–265
Yan SF, Yan SD, Ramasamy R, Schmidt AM (2009) Tempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann Med 41:408–422
Coughlan MT, Patel SK, Jerums G, Penfold SA, Nguyen TV, Sourris KC, Panagiotopoulos S, Srivastava PM, Cooper ME, Burrell LM et al (2011) Advanced glycation urinary protein-bound biomarkers and severity of diabetic nephropathy in man. Am J Nephrol 34:347–355
Friess U, Waldner M, Wahl HG, Lehmann R, Haring HU, Voelter W, Schleicher E (2003) Liquid chromatography-based determination of urinary free and total N(epsilon)-(carboxymethyl)lysine excretion in normal and diabetic subjects. J Chromatogr B Anal Technol Biomed Life Sci 794:273–280
Miyata T, Ueda Y, Horie K, Nangaku M, Tanaka S, van Ypersele de Strihou C, Kurokawa K (1998) Renal catabolism of advanced glycation end products: the fate of pentosidine. Kidney Int 53:416–422
Cooper ME (1998) Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 352:213–219
Sourris KC, Harcourt BE, Forbes JM (2009) A new perspective on therapeutic inhibition of advanced glycation in diabetic microvascular complications: common downstream endpoints achieved through disparate therapeutic approaches? Am J Nephrol 30:323–335
Li JH, Huang XR, Zhu HJ, Oldfield M, Cooper M, Truong LD, Johnson RJ, Lan HY (2004) Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J 18:176–178
Tsuchida K, Makita Z, Yamagishi S, Atsumi T, Miyoshi H, Obara S, Ishida M, Ishikawa S, Yasumura K, Koike T (1999) Suppression of transforming growth factor beta and vascular endothelial growth factor in diabetic nephropathy in rats by a novel advanced glycation end product inhibitor, OPB-9195. Diabetologia 42:579–588
Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M (2003) Advanced glycation end products inhibit de novo protein synthesis and induce TGF-beta overexpression in proximal tubular cells. Kidney Int 63:464–473
Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS et al (2003) RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 162:1123–1137
Colhoun HM, Betteridge DJ, Durrington P, Hitman G, Neil A, Livingstone S, Charlton-Menys V, Bao W, Demicco DA et al (2011) Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes 60:2379–2385
Humpert PM, Djuric Z, Kopf S, Rudofsky G, Morcos M, Nawroth PP, Bierhaus A (2007) Soluble RAGE but not endogenous secretory RAGE is associated with albuminuria in patients with type 2 diabetes. Cardiovasc Diabetol 6:9
Nin JW, Ferreira I, Schalkwijk CG, Prins MH, Chaturvedi N, Fuller JH, Stehouwer CD, Group EPCS (2009) Levels of soluble receptor for AGE are cross-sectionally associated with cardiovascular disease in type 1 diabetes, and this association is partially mediated by endothelial and renal dysfunction and by low-grade inflammation: the EURODIAB Prospective Complications Study. Diabetologia 52:705–714
Thomas MC, Soderlund J, Lehto M, Makinen VP, Moran JL, Cooper ME, Forsblom C, Groop PH, FinnDiane Study G (2011) Soluble receptor for AGE (RAGE) is a novel independent predictor of all-cause and cardiovascular mortality in type 1 diabetes. Diabetologia 54:2669–2677
Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CD (2010) Higher plasma soluble receptor for advanced glycation end products (sRAGE) levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes 59:2027–2032
Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S et al (2001) Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Investig 108:261–268
Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP et al (2009) RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. Journal of the American Society of Nephrology : JASN 20:742–752
Myint KM, Yamamoto Y, Doi T, Kato I, Harashima A, Yonekura H, Watanabe T, Shinohara H, Takeuchi M, Tsuneyama K et al (2006) RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low-molecular weight heparin. Diabetes 55:2510–2522
Tan AL, Sourris KC, Harcourt BE, Thallas-Bonke V, Penfold S, Andrikopoulos S, Thomas MC, O'Brien RC, Bierhaus A, Cooper ME et al (2010) Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. American Journal of Physiology Renal Physiology 298:F763–F770
Harcourt BE, Sourris KC, Coughlan MT, Walker KZ, Dougherty SL, Andrikopoulos S, Morley AL, Thallas-Bonke V, Chand V, Penfold SA et al (2011) Targeted reduction of advanced glycation improves renal function in obesity. Kidney Int 80:190–198
Alkhalaf A, Klooster A, van Oeveren W, Achenbach U, Kleefstra N, Slingerland RJ, Mijnhout GS, Bilo HJ, Gans RO, Navis GJ et al (2010) A double-blind, randomized, placebo-controlled clinical trial on benfotiamine treatment in patients with diabetic nephropathy. Diabetes Care 33:1598–1601
Rabbani N, Alam SS, Riaz S, Larkin JR, Akhtar MW, Shafi T, Thornalley PJ (2009) High-dose thiamine therapy for patients with type 2 diabetes and microalbuminuria: a randomised, double-blind placebo-controlled pilot study. Diabetologia 52:208–212
House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, Dresser GK, Spence JD (2010) Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. J Am Med Assoc 303:1603–1609
Watson AM, Gray SP, Jiaze L, Soro-Paavonen A, Wong B, Cooper ME, Bierhaus A, Pickering R, Tikellis C, Tsorotes D et al (2012) Alagebrium reduces glomerular fibrogenesis and inflammation beyond preventing RAGE activation in diabetic apolipoprotein E knockout mice. Diabetes 61:2105–2113
Cameron NE, Gibson TM, Nangle MR, Cotter MA (2005) Inhibitors of advanced glycation end product formation and neurovascular dysfunction in experimental diabetes. Ann N Y Acad Sci 1043:784–792
Chang KC, Liang JT, Tsai PS, Wu MS, Hsu KL (2009) Prevention of arterial stiffening by pyridoxamine in diabetes is associated with inhibition of the pathogenic glycation on aortic collagen. Br J Pharmacol 157:1419–1426
Curtis TM, Hamilton R, Yong PH, McVicar CM, Berner A, Pringle R, Uchida K, Nagai R, Brockbank S, Stitt AW (2011) Muller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia 54:690–698
Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, Baynes JW (2002) Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int 61:939–950
Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ (2003) Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 52:2110–2120
Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME et al (2003) A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res 92:785–792
Forbes JM, Thallas V, Thomas MC, Founds HW, Burns WC, Jerums G, Cooper ME (2003) The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J 17:1762–1764
Forbes JM, Yee LT, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, Thomas MC, Burns WC, Deemer EK, Thorpe SM et al (2004) Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes 53:1813–1823
Nakamura S, Makita Z, Ishikawa S, Yasumura K, Fujii W, Yanagisawa K, Kawata T, Koike T (1997) Progression of nephropathy in spontaneous diabetic rats is prevented by OPB-9195, a novel inhibitor of advanced glycation. Diabetes 46:895–899
Zheng F, He C, Cai W, Hattori M, Steffes M, Vlassara H (2002) Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev 18:224–237
Coughlan MT, Forbes JM, Cooper ME (2007) Role of the AGE crosslink breaker, Alagebrium, as a renoprotective agent in diabetes. Kidney International Supplement: S54-60.
Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, Kass DA (2007) Advanced glycation endproduct crosslink breaker (Alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens 25:577–583
Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG (2001) Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 104:1464–1470
Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, Teerlink T, Schrauwen P, Brownlee M, Stehouwer CD, Schalkwijk CG (2011) Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem 286:1374–1380
Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M (1998) Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Investig 101:1142–1147
Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnolzer M, Lasitschka F et al (2012) Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med 18:926–933
Berner AK, Brouwers O, Pringle R, Klaassen I, Colhoun L, McVicar C, Brockbank S, Curry JW, Miyata T, Brownlee M et al (2012) Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia 55:845–854
Lassègue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285:R277–R297
Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL (2005) p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol 25:512–518
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Bedard K, Lardy B, Krause KH (2007) NOX family NADPH oxidases: not just in mammals. Biochimie 89:1107–1112
Haddad JJ (2002) Science review: redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury: role for nuclear factor-κB. Crit Care 6:481–490
Kagota S, Kubota Y, Nejime N, Nakamura K, Kunitomo M, Shinozuka K (2007) Impaired effect of salt loading on nitric oxide-mediated relaxation in aortas from stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 34:48–54
Maneen MJ, Cipolla MJ (2007) Peroxynitrite diminishes myogenic tone in cerebral arteries: role of nitrotyrosine and F-actin. Am J Physiol Heart Circ Physiol 292:H1042–H1050
Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Lassègue B, Griendling KK (2007) Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27:42–48
Lassègue B, Sorescu D, Szöcs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK (2001) Novel gp91phox homologues in vascular smooth muscle cells: Nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88:888–894
Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK (2002) Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol 22:21–27
Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH (2001) Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radical Biology & Medicine 31:1456–1464
Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HHHW, Harrison DG, Griendling KK (2008) Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45:1340–1351
Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B et al (2008) Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52:1803–1809
Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH et al (2010) Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res 106:1363–1373
Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH (2006) Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580:497–504
Lee YK, Lee JY, Kim JS, Won KB, Kang HJ, Jang TJ, Tak WT, Lee JH (2009) The breakdown of preformed peritoneal advanced glycation end products by intraperitoneal alagebrium. J Korean Med Sci 24(Suppl):S189–S194
Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H et al (2005) Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112:2677–2685
Wendt MC, Daiber A, Kleschyov AL, Mülsch A, Sydow K, Schulz E, Chen K, Keaney JF Jr, Lassègue B, Walter U et al (2005) Differential effects of diabetes on the expression of the gp91 phox homologues nox1 and nox4. Free Radic Biol Med 39:381–391
Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ (2011) Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216:321–326
Judkins CP, Diep H, Broughton BRS, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR (2010) Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE −/− mice. Am J Physiol Heart Circ Physiol 298:H24–H32
Xiang FL, Lu X, Strutt B, Hill DJ, Feng Q (2010) NOX2 deficiency protects against streptozotocin-induced beta-cell destruction and development of diabetes in mice. Diabetes 59:2603–2611
You YH, Okada S, Ly S, Jandeleit-Dahm K, Barit D, Namikoshi T, Sharma K (2013) Role of Nox2 in diabetic kidney disease. American Journal of Physiology Renal Physiology 304:F840–F848
Babelova A, Avaniadi D, Jung O, Fork C, Beckmann J, Kosowski J, Weissmann N, Anilkumar N, Shah AM, Schaefer L et al (2012) Role of Nox4 in murine models of kidney disease. Free Radical Biology & Medicine 53:842–853
Block K, Gorin Y, Abboud HE (2009) Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106:14385–14390
Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE (2005) Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280:39616–39626
Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CRJ, Burns KD, Touyz RM et al (2010) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Ren Physiol 299:F1348–F1358
Chen F, Haigh S, Barman S, Fulton DJ (2012) From form to function: the role of Nox4 in the cardiovascular system. Front Physiol 3:412
Touyz RM, Montezano AC (2012) Vascular Nox4: a multifarious NADPH oxidase. Circ Res 110:1159–1161
Schmidt HH, Wingler K, Kleinschnitz C, Dusting G (2012) NOX4 is a janus-faced reactive oxygen species generating NADPH oxidase. Circ Res 111:e15–e16
Cai H (2005) NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res 96:818–822
Cai H, Griendling KK, Harrison DG (2003) The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24:471–478
Higashi T, Sano H, Saishoji T, Ikeda K, Jinnouchi Y, Kanzaki T, Morisaki N, Rauvala H, Shichiri M, Horiuchi S (1997) The receptor for advanced glycation end products mediates the chemotaxis of rabbit smooth muscle cells. Diabetes 46:463–472
Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Ludike P, Michaelis UR, Weissmann N, et al. (2012) Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res.
Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ et al (2011) Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31:1368–1376
Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS (2003) Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 285:R117–R124
Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M et al (2004) The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. Journal of the American Society of Nephrology : JASN 15:306–315
Yogi A, Mercure C, Touyz J, Callera GE, Montezano ACI, Aranha AB, Tostes RC, Reudelhuber T, Touyz RM (2008) Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension 51:500–506
Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, Thallas-Bonke V, Wingler K, Szyndralewiez C, Heitz F, et al. (2014) Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. Journal of the American Society of Nephrology: JASN.
Gorin Y, Block K (2013) Nox4 and diabetic nephropathy: with a friend like this, who needs enemies? Free Radical Biology & Medicine 61C:130–142
Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE (2003) Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Ren Physiol 285:F219–F229
Etoh T, Inoguchi T, Kakimoto M, Sonoda N, Kobayashi K, Kuroda J, Sumimoto H, Nawata H (2003) Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 46:1428–1437
New DD, Block K, Bhandhari B, Gorin Y, Abboud HE (2012) IGF-I increases the expression of fibronectin by Nox4-dependent Akt phosphorylation in renal tubular epithelial cells. American Journal of Physiology Cell Physiology 302:C122–C130
BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Gorlach A (2007) NOX5 variants are functionally active in endothelial cells. Free Radical Biology & Medicine 42:446–459
Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK (2008) Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radical Biology & Medicine 45:329–335
Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD et al (2012) Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. American Journal of Physiology Heart and Circulatory Physiology 302:H1919–H1928
Qian J, Chen F, Kovalenkov Y, Pandey D, Moseley MA, Foster MW, Black SM, Venema RC, Stepp DW, Fulton DJ (2012) Nitric oxide reduces NADPH oxidase 5 (Nox5) activity by reversible S-nitrosylation. Free Radical Biology & Medicine 52:1806–1819
Schulz E, Munzel T (2008) NOX5, a new "radical" player in human atherosclerosis? Comment on Gluzik 2008 paper. J Am Coll Cardiol 52:1810–1812
Manea A, Manea SA, Florea IC, Luca CM, Raicu M (2012) Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radical Biology & Medicine 52:1497–1507
Holterman CE, Thibodeau JF, Towaij C, Gutsol A, Montezano AC, Parks RJ, Cooper ME, Touyz RM, Kennedy CR (2013) Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. Journal of the American Society of Nephrology: JASN.
Acknowledgments
KJD is supported by a NHMRC Senior Research Fellowship and SPG is supported by a Australian Diabetes Society Early Career Fellowship.
Disclosure
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gray, S.P., Jandeleit-Dahm, K. The pathobiology of diabetic vascular complications—cardiovascular and kidney disease. J Mol Med 92, 441–452 (2014). https://doi.org/10.1007/s00109-014-1146-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1146-1