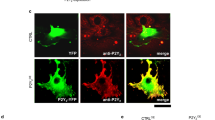
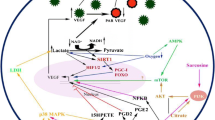
Abstract
The group of dinucleoside polyphosphates encompasses a large number of molecules consisting of two nucleosides which are connected by a phosphate chain of variable length. While the receptors activated by dinucleoside polyphosphates as well as their degradation have been studied in detail, its biosynthesis has not been elucidated so far. Since endothelial cells released the dinucleoside polyphosphate uridine adenosine tetraphosphate (Up4A), we tested cytosolic proteins of human endothelial cells obtained from dermal vessels elicited for enzymatic activity. When incubated with ADP and UDP, these cells showed increasing concentrations of Up4A. The underlying enzyme was isolated by chromatography and the mass spectrometric analysis revealed that the enzymatic activity was caused by the vascular endothelial growth factor receptor 2 (VEGFR2). Since VEGFR2 but neither VEGFR1 nor VEGFR3 were capable to synthesise dinucleoside polyphosphates, Tyr-1175 of VEGFR2 is most likely essential for the enzymatic activity of interest. Further, VEGFR2-containing cells like HepG2, THP-1 and RAW264.7 were capable of synthesising dinucleoside polyphosphates. VEGFR2-transfected HEK 293T/17 but not native HEK 293T/17 cells synthesised dinucleoside polyphosphates in vivo too. The simultaneous biosynthesis of dinucleoside polyphosphates could amplify the response to VEGF, since dinucleoside polyphosphates induce cellular growth via P2Y purinergic receptors. Thus the biosynthesis of dinucleoside polyphosphates by VEGFR2 may enhance the proliferative response to VEGF. Given that VEGFR2 is primarily expressed in endothelial cells, the biosynthesis of dinucleoside polyphosphates is mainly located in the vascular system. Since the vasculature is also the main site of action of dinucleoside polyphosphates, activating vascular purinoceptors, blood vessels appear as an autocrine system with respect to dinucleoside polyphosphates. We conclude that VEGFR2 receptor is capable of synthesising dinucleoside polyphosphates. These mediators may modulate the effects of VEGFR2 due to their proliferative effects.




Similar content being viewed by others
References
Wiedon A, Tolle M, Bastine J, Schuchardt M, Huang T, Jankowski V, Jankowski J, Zidek W, van der Giet M (2012) Uridine adenosine tetraphosphate (Up4A) is a strong inductor of smooth muscle cell migration via activation of the P2Y2 receptor and cross-communication to the PDGF receptor. Biochem Biophys Res Commun 417:1035–1040
Jankowski V, Guenthner T, Herget-Rosenthal S, Zidek W, Jankowski J (2009) Dinucleoside polyphosphates and uremia. Semin Dial 22:396–399
Jankowski V, van der Giet M, Mischak H, Morgan M, Zidek W, Jankowski J (2009) Dinucleoside polyphosphates: strong endogenous agonists of the purinergic system. Br J Pharmacol 157:1142–1153
Rapaport E, Zamecnik PC (1976) Presence of diadenosine 5′,5″ -P1, P4-tetraphosphate (Ap4A) in mamalian cells in levels varying widely with proliferative activity of the tissue: a possible positive "pleiotypic activator". Proc Natl Acad Sci USA 73:3984–3988
Flodgaard H, Klenow H (1982) Abundant amounts of diadenosine 5′,5″′-P1, P4-tetraphosphate are present and releasable, but metabolically inactive, in human platelets. Biochem J 208:737–742
Lüthje J, Ogilvie A (1983) The presence of diadenosine 5′,5′″-P1, P3-triphosphate (Ap3A) in human platelets. Biochem Biophys Res Commun 115:253–260
Jankowski J, Tepel M, van der Giet M, Tente IM, Henning L, Junker R, Zidek W, Schlüter H (1999) Identification and characterization of P1, P7-diadenosine-5′-heptaphosphate from human platelets. J Biol Chem 274:23926–23931
Schlüter H, Offers E, Brüggemann G, van der Giet M, Tepel M, Nordhoff E, Karas M, Spieker C, Witzel H, Zidek W (1994) Diadenosine phosphates and the physiological control of blood pressure. Nature 367:186–188
Yegutkin G, Jankowski J, Jalkanen S, Gunthner T, Zidek W, Jankowski V (2008) Dinucleotide polyphosphates contribute to purinergic signalling via inhibition of adenylate kinase activity. Biosci Rep 28:189–194
Yelovitch S, Camden J, Weisman GA, Fischer B (2012) Boranophosphate isoster controls P2Y-receptor subtype selectivity and metabolic stability of dinucleoside polyphosphate analogues. J Med Chem 55:437–448. doi:10.1021/jm2013198
Matsumoto T, Tostes RC, Webb RC (2012) Alterations in vasoconstrictor responses to the endothelium-derived contracting factor uridine adenosine tetraphosphate are region specific in DOCA-salt hypertensive rats. Pharmacol Res 65:81–90
Gui Y, He G, Walsh MP, Zheng XL (2011) Signaling mechanisms mediating uridine adenosine tetraphosphate-induced proliferation of human vascular smooth muscle cells. J Cardiovasc Pharmacol 58:654–662. doi:10.1097/FJC.0b013e318231e929
Jankowski V, Tölle M, Vanholder R, Schönfelder G, van der Giet M, Henning L, Schlüter H, Paul M, Zidek W, Jankowski J (2005) Identification of uridine adenosine tetraphosphate (Up4A) as an endothelium-derived vasoconstrictive factor. Nat Med 11:223–227
Zhou Z, Merkus D, Cheng C, Duckers HJ, Jan Danser AH, Duncker DJ (2012) Uridine adenosine tetraphosphate is a novel vasodilator in the coronary microcirculation which acts through purinergic P1 but not P2 receptors. Pharmacol Res 67:10–17
Matsumoto T, Tostes RC, Webb RC (2011) The role of uridine adenosine tetraphosphate in the vascular system. Adv Pharmacol Sci. doi:10.1155/2011/435132
Jankowski V, Vanholder R, van der Giet M, Tölle M, Karadogan S, Gobom J, Furkert J, Oksche A, Krause E, Tran TN et al (2007) Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arterioscler Thromb Vasc Biol 27:297–302
Gobom J, Schuerenberg M, Mueller M, Theiss D, Lehrach H, Nordhoff E (2001) Alpha-cyano-4-hydroxycinnamic acid affinity sample preparation. A protocol for MALDI-MS peptide analysis in proteomics. Anal Chem 73:434–438
Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551–3567. doi:10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2
Consortium TU (2011) Ongoing and future developments at the Universal Protein Resource. Nucleic Acids Res 39:D214–219
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lehmann K, Janda E, Pierreux CE, Rytomaa M, Schulze A, McMahon M, Hill CS, Beug H, Downward J (2000) Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev 14:2610–2622
Tanaka K, Kawakami T, Tateishi K, Yashiroda H, Chiba T (2001) Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway. Biochimie 83:351–356
Ma DJ, Li SJ, Wang LS, Dai J, Zhao SL, Zeng R (2009) Temporal and spatial profiling of nuclei-associated proteins upon TNF-alpha/NF-kappaB signaling. Cell Res 19:651–664
Zhu S, Browning DD, White RE, Fulton D, Barman SA (2009) Mutation of protein kinase C phosphorylation site S1076 on alpha-subunits affects BK(Ca) channel activity in HEK-293 cells. Am J Physiol Lung Cell Mol Physiol 297:L758–766
Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J (2004) Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A 101:13489–13494. doi:10.1073/pnas.0405659101
Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 66:11851–11858
Zhang S, Cao Z, Tian H, Shen G, Ma Y, Xie H, Liu Y, Zhao C, Deng S, Yang Y et al (2011) SKLB1002, a novel potent inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and tumor growth in vivo. Clin Cancer Res 17:4439–4450
Spiekermann K, Faber F, Voswinckel R, Hiddemann W (2002) The protein tyrosine kinase inhibitor SU5614 inhibits VEGF-induced endothelial cell sprouting and induces growth arrest and apoptosis by inhibition of c-kit in AML cells. Exp Hematol 30:767–773
Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA (2008) Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 68:4340–4346
Girling JE, Rogers PA (2009) Regulation of endometrial vascular remodelling: role of the vascular endothelial growth factor family and the angiopoietin-TIE signalling system. Reproduction 138:883–893
Sase H, Watabe T, Kawasaki K, Miyazono K, Miyazawa K (2009) VEGFR2-PLCgamma1 axis is essential for endothelial specification of VEGFR2+ vascular progenitor cells. J Cell Sci 122:3303–3311
Holmqvist K, Cross MJ, Rolny C, Hagerkvist R, Rahimi N, Matsumoto T, Claesson-Welsh L, Welsh M (2004) The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem 279:22267–22275. doi:10.1074/jbc.M312729200
Jankowski V, Meyer AA, Schlattmann P, Gui Y, Zheng XL, Stamcou I, Radtke K, Anh Tran TN, van der Giet M, Tölle M et al (2007) Increased Uridine Adenosine Tetraphosphate Concentrations in Plasma of Juvenile Hypertensives. Arterioscler Thromb Vasc Biol 27:1776–1781
Acknowledgements
This study was supported by a grant of the German Research Foundation (DFG, Ja-972 /11-1/2), a grant from the Federal Ministry of Education and Research (01GR080701/09), grant FP7-HEALTH-2009-2.4.5-2 to “SysKid” (grant agreement 241544), “Mascara” (grant agreement 278249) from the European Union and by the Peter und Traudl Engelhorn Stiftung. We thank B. Egbers and D. Jacobi for excellent technical assistance.
Author information
Authors and Affiliations
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 185 kb)
Rights and permissions
About this article
Cite this article
Jankowski, V., Schulz, A., Kretschmer, A. et al. The enzymatic activity of the VEGFR2 receptor for the biosynthesis of dinucleoside polyphosphates. J Mol Med 91, 1095–1107 (2013). https://doi.org/10.1007/s00109-013-1036-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-013-1036-y