Abstract
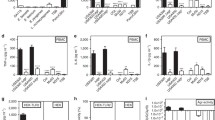
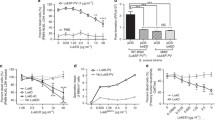
Toll-like receptors (TLRs) are crucial for our host defense against microbial infections. TLR2 is especially important to fight bacterial infections, as it specifically recognizes bacterial lipoproteins of both Gram-positive and Gram-negative origin. Present on a variety of immune cells, TLR2 is critical for host protection against several bacterial infections, including those caused by Staphylococcus aureus. This major human pathogen causes increasing health care problems due to its increased resistance to antibiotics. S. aureus secretes a wide variety of proteins that inhibit innate immune responses. Recently, several staphylococcal superantigen-like proteins (SSLs) have been described to mediate immune evasive properties. Here, we describe that SSL3 specifically binds and inhibits TLR2 activation on human and murine neutrophils and monocytes. Through binding of the extracellular TLR2 domain, SSL3 inhibits IL-8 production by HEK cells expressing TLR1/2 and TLR2/6 dimers, stimulated with their specific ligands. The SSL3–TLR2 interaction is partially glycan dependent as binding of SSL3 to TLR2 is affected upon removal of sialic acid residues. Moreover, the SSL3(R308A) mutant lacking glycan-binding properties shows lower TLR2 inhibition. An SSL3 mutant, lacking the N-terminal 126 amino acids, still retains full TLR2 inhibiting activity. Of other SSLs tested, only SSL4, which shares the highest homology with SSL3, blocks TLR2 activation. SSL3 is the first-described bacterial protein that blocks TLR2 activation through direct extracellular interaction with the receptor. This unique function of SSL3 adds to the arsenal of immune evasive molecules that S. aureus can employ to subvert both innate and adaptive immunity.









Similar content being viewed by others
References
Arpaia N, Barton GM (2011) Toll-like receptors: key players in antiviral immunity. Curr Opin Virol 1:447–454
Bardoel BW, Strijp JA (2012) Molecular battle between host and bacterium: recognition in innate immunity. J Mol Recognit 24:1077–1086
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Chang ZL (2010) Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res 59:791–808
Iwasaki A, Medzhitov R (2004) Toll-like receptor control of the adaptive immune responses. Nat Immunol 5:987–995
Krutzik SR, Sieling PA, Modlin RL (2001) The role of Toll-like receptors in host defense against microbial infection. Curr Opin Immunol 13:104–108
Medzhitov R (2001) Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145
Zahringer U, Lindner B, Inamura S, Heine H, Alexander C (2008) TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205–224
Jin MS, Lee JO (2008) Structures of the toll-like receptor family and its ligand complexes. Immunity 29:182–191
Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071–1082
Schenk M, Belisle JT, Modlin RL (2009) TLR2 looks at lipoproteins. Immunity 31:847–849
Archer KA, Roy CR (2006) MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect Immun 74:3325–3333
Dickinson GS, Piccone H, Sun G, Lien E, Gatto L, Alugupalli KR (2010) Toll-like receptor 2 deficiency results in impaired antibody responses and septic shock during Borrelia hermsii infection. Infect Immun 78:4579–4588
Mancuso G, Midiri A, Beninati C, Biondo C, Galbo R, Akira S, Henneke P, Golenbock D, Teti G (2004) Dual role of TLR2 and myeloid differentiation factor 88 in a mouse model of invasive group B streptococcal disease. J Immunol 172:6324–6329
Takeuchi O, Hoshino K, Akira S (2000) Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165:5392–5396
Stenzel W, Soltek S, Sanchez-Ruiz M, Akira S, Miletic H, Schluter D, Deckert M (2008) Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am J Pathol 172:132–145
Rooijakkers SH, van Kessel KP, van Strijp JA (2005) Staphylococcal innate immune evasion. Trends Microbiol 13:596–601
Lina G, Bohach GA, Nair SP, Hiramatsu K, Jouvin-Marche E, Mariuzza R (2004) Standard nomenclature for the superantigens expressed by Staphylococcus. J Infect Dis 189:2334–2336
Bestebroer J, Poppelier MJ, Ulfman LH, Lenting PJ, Denis CV, van Kessel KP, van Strijp JA, de Haas CJ (2007) Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 109:2936–2943
Walenkamp AM, Bestebroer J, Boer IG, Kruizinga R, Verheul HM, van Strijp JA, de Haas CJ (2010) Staphylococcal SSL5 binding to human leukemia cells inhibits cell adhesion to endothelial cells and platelets. Cell Oncol 32:1–10
Bestebroer J, van Kessel KP, Azouagh H, Walenkamp AM, Boer IG, Romijn RA, van Strijp JA, de Haas CJ (2009) Staphylococcal SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. Blood 113:328–337
de Haas CJ, Weeterings C, Vughs MM, de Groot PG, Van Strijp JA, Lisman T (2009) Staphylococcal superantigen-like 5 activates platelets and supports platelet adhesion under flow conditions, which involves glycoprotein Ibalpha and alpha IIb beta 3. J Thromb Haemost 7:1867–1874
Itoh S, Hamada E, Kamoshida G, Takeshita K, Oku T, Tsuji T (2010) Staphylococcal superantigen-like protein 5 inhibits matrix metalloproteinase 9 from human neutrophils. Infect Immun 78:3298–3305
Bestebroer J, Aerts PC, Rooijakkers SH, Pandey MK, Kohl J, van Strijp JA, de Haas CJ (2010) Functional basis for complement evasion by staphylococcal superantigen-like 7. Cell Microbiol 12:1506–1516
Walenkamp AM, Boer IG, Bestebroer J, Rozeveld D, Timmer-Bosscha H, Hemrika W, van Strijp JA, de Haas CJ (2009) Staphylococcal superantigen-like 10 inhibits CXCL12-induced human tumor cell migration. Neoplasia 11:333–344
Itoh S, Hamada E, Kamoshida G, Yokoyama R, Takii T, Onozaki K, Tsuji T (2010) Staphylococcal superantigen-like protein 10 (SSL10) binds to human immunoglobulin G (IgG) and inhibits complement activation via the classical pathway. Mol Immunol 47:932–938
Patel D, Wines BD, Langley RJ, Fraser JD (2010) Specificity of staphylococcal superantigen-like protein 10 toward the human IgG1 Fc domain. J Immunol 184:6283–6292
Baker HM, Basu I, Chung MC, Caradoc-Davies T, Fraser JD, Baker EN (2007) Crystal structures of the staphylococcal toxin SSL5 in complex with sialyl Lewis X reveal a conserved binding site that shares common features with viral and bacterial sialic acid binding proteins. J Mol Biol 374:1298–1308
Chung MC, Wines BD, Baker H, Langley RJ, Baker EN, Fraser JD (2007) The crystal structure of staphylococcal superantigen-like protein 11 in complex with sialyl Lewis X reveals the mechanism for cell binding and immune inhibition. Mol Microbiol 66:1342–1355
Bardoel BW, Hartsink D, Vughs MM, de Haas CJ, van Strijp JA, van Kessel KP (2012) Identification of an immunomodulating metalloprotease of Pseudomonas aeruginosa (IMPa). Cell Microbiol 14:902–913
Haas PJ, de Haas CJ, Kleibeuker W, Poppelier MJ, van Kessel KP, Kruijtzer JA, Liskamp RM, van Strijp JA (2004) N-terminal residues of the chemotaxis inhibitory protein of Staphylococcus aureus are essential for blocking formylated peptide receptor but not C5a receptor. J Immunol 173:5704–5711
Bardoel BW, van der Ent S, Pel MJ, Tommassen J, Pieterse CM, van Kessel KP, van Strijp JA (2011) Pseudomonas evades immune recognition of flagellin in both mammals and plants. PLoS Pathog 7:e1002206
Jongerius I, Kohl J, Pandey MK, Ruyken M, van Kessel KP, van Strijp JA, Rooijakkers SH (2007) Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med 204:2461–2471
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J (2007) Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol 8:610–618
Jimenez-Dalmaroni MJ, Xiao N, Corper AL, Verdino P, Ainge GD, Larsen DS, Painter GF, Rudd PM, Dwek RA, Hoebe K et al (2009) Soluble CD36 ectodomain binds negatively charged diacylglycerol ligands and acts as a co-receptor for TLR2. PLoS One 4:e7411
Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ (2005) Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol 170:477–485
Gerold G, Ajaj KA, Bienert M, Laws HJ, Zychlinsky A, de Diego JL (2008) A Toll-like receptor 2-integrin beta3 complex senses bacterial lipopeptides via vitronectin. Nat Immunol 9:761–768
Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S et al (2004) Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest 113:1473–1481
Weber AN, Morse MA, Gay NJ (2004) Four N-linked glycosylation sites in human toll-like receptor 2 cooperate to direct efficient biosynthesis and secretion. J Biol Chem 279:34589–34594
Fraser JD, Proft T (2008) The bacterial superantigen and superantigen-like proteins. Immunol Rev 225:226–243
O'Neill LA, Bryant CE, Doyle SL (2009) Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev 61:177–197
Benson MA, Lilo S, Wasserman GA, Thoendel M, Smith A, Horswill AR, Fraser J, Novick RP, Shopsin B, Torres VJ (2011) Staphylococcus aureus regulates the expression and production of the staphylococcal superantigen-like secreted proteins in a Rot-dependent manner. Mol Microbiol 81:659–675
Acknowledgements
These studies were performed under the Immuno Valley ALTANT program funded by the Netherlands Ministry of Agriculture, Nature, and Food Quality and, later on, Netherlands Ministry of Economic Affairs, Agriculture, and Innovation. The authors thank Dr. Mark de Been for his help in constructing the phylogenetic trees.
Disclosure statement
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 301 kb)
Rights and permissions
About this article
Cite this article
Bardoel, B.W., Vos, R., Bouman, T. et al. Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3. J Mol Med 90, 1109–1120 (2012). https://doi.org/10.1007/s00109-012-0926-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0926-8