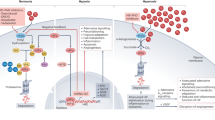
Abstract
Endothelial-derived nitric oxide (NO) is classically viewed as a regulator of vasomotor tone. NO plays an important role in regulating O2 delivery through paracrine control of vasomotor tone locally and cardiovascular and respiratory responses centrally. Very soon after the cloning and functional characterization of the endothelial nitric oxide synthase (eNOS), studies on the interaction between O2 and NO made the paradoxical finding that hypoxia led to decreases in eNOS expression and function. Why would decreases in O2 content in tissues elicit a loss of a potent endothelial-derived vasodilator? We now know that restricting our view of NO as a regulator of vasomotor tone or blood pressure limited deeper levels of mechanistic insight. Exciting new studies indicate that functional interactions between NO and O2 exhibit profound complexity and are relevant to diseases states, especially those associated with hypoxia in tissues. NOS isoforms catalytically require O2. Hypoxia regulates steady-state expression of the mRNA and protein abundance of the NOS enzymes. Animals genetically deficient in NOS isoforms have perturbations in their ability to adapt to changes in O2 supply or demand. Most interestingly, the intracellular pathways for O2 sensing that evolved to ensure an appropriate balance of O2 delivery and utilization intersect with NO signaling networks. Recent studies demonstrate that hypoxia-inducible factor (HIF) stabilization and transcriptional activity is achieved through two parallel pathways: (1) a decrease in O2-dependent prolyl hydroxylation of HIF and (2) S-nitrosylation of HIF pathway components. Recent findings support a role for S-nitrosothiols as hypoxia-mimetics in certain biological and/or disease settings, such as living at high altitude, exposure to small molecules that can bind NO, or anemia.




Similar content being viewed by others
References
Semenza GL (2011) Oxygen sensing, homeostasis, and disease. N Engl J Med 365:537–547
Tsui AK, Marsden PA, Mazer CD, Adamson SL, Henkelman RM, Ho JJ, Wilson DF, Heximer SP, Connelly KA, Bolz SS et al (2011) Priming of hypoxia-inducible factor by neuronal nitric oxide synthase is essential for adaptive responses to severe anemia. Proc Natl Acad Sci USA 108:17544–17549
Beall CM (2007) Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA 104(Suppl 1):8655–8660
Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ et al (2007) Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA 104:17593–17598
Hoit BD, Dalton ND, Erzurum SC, Laskowski D, Strohl KP, Beall CM (2005) Nitric oxide and cardiopulmonary hemodynamics in Tibetan highlanders. J Appl Physiol 99:1796–1801
Janocha AJ, Koch CD, Tiso M, Ponchia A, Doctor A, Gibbons L, Gaston B, Beall CM, Erzurum SC (2011) Nitric oxide during altitude acclimatization. N Engl J Med 365:1942–1944
Lima B, Forrester MT, Hess DT, Stamler JS (2010) S-nitrosylation in cardiovascular signaling. Circ Res 106:633–646
Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB et al (2010) Genetic evidence for high-altitude adaptation in Tibet. Science 329:72–75
Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M et al (2010) Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107:11459–11464
Moncada S, Higgs A (1993) The L-arginine-nitric oxide pathway. N Engl J Med 329:2002–2012
Michel T, Feron O (1997) Nitric oxide synthases: which, where, how, and why? J Clin Investig 100:2146–2152
Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH (1991) Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 351:714–718
Lowenstein CJ, Glatt CS, Bredt DS, Snyder SH (1992) Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci USA 89:6711–6715
Marsden PA, Schappert KT, Chen HS, Flowers M, Sundell CL, Wilcox JN, Lamas S, Michel T (1992) Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett 307:287–293
Wong GK, Marsden PA (1996) Nitric oxide synthases: regulation in disease. Nephrol, Dial, Transplant: Off Publ Eur Dial Transplant Assoc-Eur Renal Assoc 11:215–220
Lamas S, Perez-Sala D, Moncada S (1998) Nitric oxide: from discovery to the clinic. Trends Pharmacol Sci 19:436–438
Ignarro LJ (1999) Nitric oxide: a unique endogenous signaling molecule in vascular biology. Biosci Rep 19:51–71
Li H, Forstermann U (2000) Nitric oxide in the pathogenesis of vascular disease. J Pathol 190:244–254
Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA (1997) Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol 17:2479–2488
Berchner-Pfannschmidt U, Tug S, Kirsch M, Fandrey J (2010) Oxygen-sensing under the influence of nitric oxide. Cell Signal 22:349–356
Brahimi-Horn MC, Pouyssegur J (2007) Oxygen, a source of life and stress. FEBS Lett 581:3582–3591
Brahimi-Horn MC, Chiche J, Pouyssegur J (2007) Hypoxia and cancer. J Mol Med 85:1301–1307
Bunn HF, Poyton RO (1996) Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev 76:839–885
Hochachka PW, Monge C (2000) Evolution of human hypoxia tolerance physiology. Adv Exp Med Biol 475:25–43
Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW et al (2006) Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J 25:1114–1125
Shih SC, Claffey KP (1998) Hypoxia-mediated regulation of gene expression in mammalian cells. Int J Exp Pathol 79:347–357
Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL (2005) Acute oxygen-sensing mechanisms. N Engl J Med 353:2042–2055
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92:5510–5514
Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ (1996) The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J Biol Chem 271:15117–15123
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408
Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129:111–122
Ullah MS, Davies AJ, Halestrap AP (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281:9030–9037
Ouiddir A, Planes C, Fernandes I, VanHesse A, Clerici C (1999) Hypoxia upregulates activity and expression of the glucose transporter GLUT1 in alveolar epithelial cells. Am J Respir Cell Mol Biol 21:710–718
Evans AJ, Russell RC, Roche O, Burry TN, Fish JE, Chow VW, Kim WY, Saravanan A, Maynard MA, Gervais ML et al (2007) VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol Cell Biol 27:157–169
Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, Hast BE, Hanna SC, Wondergem B, Furge KA et al (2009) Regulation of endocytosis via the oxygen-sensing pathway. Nat Med 15:319–324
Tian H, McKnight SL, Russell DW (1997) Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11:72–82
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC (2003) Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23:9361–9374
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ (2005) Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel–Lindau-associated renal cell carcinoma. Mol Cell Biol 25:5675–5686
Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ (2009) Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 284:16767–16775
Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR (2009) Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA 106:1199–1204
Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL et al (2011) Hypoxia-inducible factor 2alpha (HIF-2alpha) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci USA 108:3065–3070
Branco-Price C, Zhang N, Schnelle M, Evans C, Katschinski DM, Liao D, Ellies L, Johnson RS (2012) Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer cell 21:52–65
Kaelin WG Jr (2007) The von Hippel–Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res: An Off J Am Assoc Cancer Res 13:680s–684s
Kallio PJ, Wilson WJ, O’Brien S, Makino Y, Poellinger L (1999) Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem 274:6519–6525
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A et al (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54
Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337–1340
Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J 20:5197–5206
Huang LE, Gu J, Schau M, Bunn HF (1998) Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 95:7987–7992
O’Rourke JF, Tian YM, Ratcliffe PJ, Pugh CW (1999) Oxygen-regulated and transactivating domains in endothelial PAS protein 1: comparison with hypoxia-inducible factor-1alpha. J Biol Chem 274:2060–2071
Ivan M, Kaelin WG Jr (2001) The von Hippel–Lindau tumor suppressor protein. Curr Opin Genet Dev 11:27–34
Kaelin WG Jr (2008) The von Hippel–Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8:865–873
Safran M, Kaelin WG Jr (2003) HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Investig 111:779–783
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY et al (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12:149–162
Ginouves A, Ilc K, Macias N, Pouyssegur J, Berra E (2008) PHDs overactivation during chronic hypoxia “desensitizes” HIFalpha and protects cells from necrosis. Proc Natl Acad Sci USA 105:4745–4750
Liu W, Xin H, Eckert DT, Brown JA, Gnarra JR (2011) Hypoxia and cell cycle regulation of the von Hippel–Lindau tumor suppressor. Oncogene 30:21–31
Foster MW, McMahon TJ, Stamler JS (2003) S-nitrosylation in health and disease. Trends Mol Med 9:160–168
Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6:150–166
Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, Law L, Hester LD, Snyder SH (2010) GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol 12:1094–1100
Benhar M, Forrester MT, Stamler JS (2009) Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 10:721–732
Gaston BM, Carver J, Doctor A, Palmer LA (2003) S-nitrosylation signaling in cell biology. Mol Interv 3:253–263
Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY (2007) Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 26:63–74
Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B (2007) S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Investig 117:2592–2601
Chowdhury R, Flashman E, Mecinovic J, Kramer HB, Kessler BM, Frapart YM, Boucher JL, Clifton IJ, McDonough MA, Schofield CJ (2011) Studies on the reaction of nitric oxide with the hypoxia-inducible factor prolyl hydroxylase domain 2 (EGLN1). J Mol Biol 410:268–279
Berchner-Pfannschmidt U, Yamac H, Trinidad B, Fandrey J (2007) Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J Biol Chem 282:1788–1796
Marsden PA (2007) Low-molecular-weight S-nitrosothiols and blood vessel injury. J Clin Investig 117:2377–2380
Jia L, Bonaventura C, Bonaventura J, Stamler JS (1996) S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380:221–226
McMahon TJ, Exton Stone A, Bonaventura J, Singel DJ, Solomon Stamler J (2000) Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem 275:16738–16745
Kosaka H, Seiyama A (1996) Physiological role of nitric oxide as an enhancer of oxygen transfer from erythrocytes to tissues. Biochem Biophys Res Commun 218:749–752
Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM et al (2009) Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci USA 106:6297–6302
Singel DJ, Stamler JS (2005) Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67:99–145
Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B (2001) S-nitrosothiols signal the ventilatory response to hypoxia. Nature 413:171–174
Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR (2001) Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537:613–621
Casey DP, Curry TB, Wilkins BW, Joyner MJ (2011) Nitric oxide-mediated vasodilation becomes independent of beta-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol 110:687–694
Naghshin J, McGaffin KR, Witham WG, Mathier MA, Romano LC, Smith SH, Janczewski AM, Kirk JA, Shroff SG, O’Donnell CP (2009) Chronic intermittent hypoxia increases left ventricular contractility in C57BL/6J mice. J Appl Physiol 107:787–793
Baloglu E, Reingruber T, Bartsch P, Mairbaurl H (2011) beta2-Adrenergics in hypoxia desensitize receptors but blunt inhibition of reabsorption in rat lungs. Am J Respir Cell Mol Biol 45:1059–1068
Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA et al (2007) Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 129:511–522
Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ, Stamler JS (2008) S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol Cell 31:395–405
Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS et al (2009) A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361:2019–2032
Schofield CJ, Ratcliffe PJ (2004) Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5:343–354
Deem S, Hedges RG, McKinney S, Polissar NL, Alberts MK, Swenson ER (1999) Mechanisms of improvement in pulmonary gas exchange during isovolemic hemodilution. J Appl Physiol 87:132–141
Ward ME, Toporsian M, Scott JA, Teoh H, Govindaraju V, Quan A, Wener AD, Wang G, Bevan SC, Newton DC et al (2005) Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J Clin Investig 115:3128–3139
Newton DC, Bevan SC, Choi S, Robb GB, Millar A, Wang Y, Marsden PA (2003) Translational regulation of human neuronal nitric-oxide synthase by an alternatively spliced 5′-untranslated region leader exon. J Biol Chem 278:636–644
Wang Y, Newton DC, Marsden PA (1999) Neuronal NOS: gene structure, mRNA diversity, and functional relevance. Crit Rev Neurobiol 13:21–43
Wang Y, Newton DC, Miller TL, Teichert AM, Phillips MJ, Davidoff MS, Marsden PA (2002) An alternative promoter of the human neuronal nitric oxide synthase gene is expressed specifically in Leydig cells. Am J Pathol 160:369–380
Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S (2004) Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem 279:36167–36170
Dawson VL, Dawson TM (1996) Nitric oxide neurotoxicity. J Chem Neuroanat 10:179–190
Semenza GL (2005) New insights into nNOS regulation of vascular homeostasis. J Clin Investig 115:2976–2978
Robinson MA, Baumgardner JE, Otto CM (2011) Oxygen-dependent regulation of nitric oxide production by inducible nitric oxide synthase. Free Radic Biol Med 51:1952–1965
Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L (1995) A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. The J Exp Med 182:1683–1693
Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS (2010) Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev 24:491–501
Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J et al (2006) Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA 103:18154–18159
Chan GC, Fish JE, Mawji IA, Leung DD, Rachlis AC, Marsden PA (2005) Epigenetic basis for the transcriptional hyporesponsiveness of the human inducible nitric oxide synthase gene in vascular endothelial cells. J Immunol 175:3846–3861
Matouk CC, Marsden PA (2008) Epigenetic regulation of vascular endothelial gene expression. Circ Res 102:873–887
McQuillan LP, Leung GK, Marsden PA, Kostyk SK, Kourembanas S (1994) Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol 267:H1921–H1927
Ostergaard L, Stankevicius E, Andersen MR, Eskildsen-Helmond Y, Ledet T, Mulvany MJ, Simonsen U (2007) Diminished NO release in chronic hypoxic human endothelial cells. Am J Physiol Heart Circ Physiol 293:H2894–H2903
Fish JE, Matouk CC, Yeboah E, Bevan SC, Khan M, Patil K, Ohh M, Marsden PA (2007) Hypoxia-inducible expression of a natural cis-antisense transcript inhibits endothelial nitric-oxide synthase. J Biol Chem 282:15652–15666
Fish JE, Yan MS, Matouk CC, St Bernard R, Ho JJ, Gavryushova A, Srivastava D, Marsden PA (2010) Hypoxic repression of endothelial nitric-oxide synthase transcription is coupled with eviction of promoter histones. J Biol Chem 285:810–826
Robb GB, Carson AR, Tai SC, Fish JE, Singh S, Yamada T, Scherer SW, Nakabayashi K, Marsden PA (2004) Post-transcriptional regulation of endothelial nitric-oxide synthase by an overlapping antisense mRNA transcript. J Biol Chem 279:37982–37996
Shaul PW, Wells LB, Horning KM (1993) Acute and prolonged hypoxia attenuate endothelial nitric oxide production in rat pulmonary arteries by different mechanisms. J Cardiovasc Pharmacol 22:819–827
Ziesche R, Petkov V, Williams J, Zakeri SM, Mosgoller W, Knofler M, Block LH (1996) Lipopolysaccharide and interleukin 1 augment the effects of hypoxia and inflammation in human pulmonary arterial tissue. Proc Natl Acad Sci USA 93:12478–12483
Justice JM, Tanner MA, Myers PR (2000) Endothelial cell regulation of nitric oxide production during hypoxia in coronary microvessels and epicardial arteries. J Cell Physiol 182:359–365
Quinlan TR, Li D, Laubach VE, Shesely EG, Zhou N, Johns RA (2000) eNOS-deficient mice show reduced pulmonary vascular proliferation and remodeling to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 279:L641–L650
Murata T, Sato K, Hori M, Ozaki H, Karaki H (2002) Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 277:44085–44092
Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM (1997) Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res 81:34–41
Fagan KA, Fouty BW, Tyler RC, Morris KG Jr, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL et al (1999) The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Investig 103:291–299
Coulet F, Nadaud S, Agrapart M, Soubrier F (2003) Identification of hypoxia-response element in the human endothelial nitric-oxide synthase gene promoter. J Biol Chem 278:46230–46240
Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI (2007) Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol 171:1691–1704
Teichert AM, Scott JA, Robb GB, Zhou YQ, Zhu SN, Lem M, Keightley A, Steer BM, Schuh AC, Adamson SL et al (2008) Endothelial nitric oxide synthase gene expression during murine embryogenesis: commencement of expression in the embryo occurs with the establishment of a unidirectional circulatory system. Circ Res 103:24–33
Resta TC, Chicoine LG, Omdahl JL, Walker BR (1999) Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol 276:H699–H708
Le Cras TD, Xue C, Rengasamy A, Johns RA (1996) Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am J Physiol 270:L164–L170
Quinlan TR, Laubach V, Zhou N, Johns RA (1998) Alterations in nitric oxide synthase isoform expression in NOS knockout mice exposed to normoxia or hypoxia. Chest 114:53S–55S
Hagen T, Taylor CT, Lam F, Moncada S (2003) Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science 302:1975–1978
Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE (1999) AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443:285–289
Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T (2006) Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem 281:151–157
Erwin PA, Lin AJ, Golan DE, Michel T (2005) Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 280:19888–19894
Acknowledgments
J.J.H. is a recipient of an Ontario Graduate Scholarship. H.S.M. is a recipient of a CIHR Training Program in Regenerative Medicine Fellowship. P.A.M. holds the Keenan Chair in Medical Research at St. Michael’s Hospital and the University of Toronto and is supported by a grant from the Canadian Institute of Health research (CIHR MOP 79475).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ho, J.J.D., Man, H.S.J. & Marsden, P.A. Nitric oxide signaling in hypoxia. J Mol Med 90, 217–231 (2012). https://doi.org/10.1007/s00109-012-0880-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0880-5