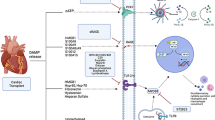
Abstract
Extensive cell death with consecutive release of danger signals can cause immune-mediated tissue destruction. The abundance of cell death is likely to determine the relevance of the danger signals as physiological mechanisms that counteract immune activation may be overruled. Such constellation is conceivable in chemo-/radiotherapy-induced tissue damage, reperfusion injury, trauma, and severe infection. Studies on graft-versus-host disease (GvHD) development have to consider the effects of chemo-/radiotherapy-related tissue damage leading to the release of exogenous and endogenous danger signals. Our previous work has demonstrated a role for adenosine-5′-triphosphate (ATP) as an endogenous danger signal in GvHD. Besides ATP, uric acid or soluble extracellular matrix components are functional danger signals that activate the NLRP3 inflammasome when released from dying cells or from extracellular matrix. In contrast to sterile inflammation, GvHD is more complex since bacterial components that leak through damaged intestinal barriers and the skin can activate pattern recognition receptors and directly contribute to GvHD pathogenesis. These exogenous danger signals transmit immune activation via toll-like receptors and NOD-like receptors of the innate immune system. This review covers both the impact of endogenous and exogenous danger signals activating innate immunity in GvHD.

Similar content being viewed by others
Abbreviations
- APCs:
-
Antigen-presenting cells
- ASC:
-
Apoptosis-associated speck-like protein containing caspase activation and recruitment domain
- ATP:
-
Adenosine-5′-triphosphate
- CARD:
-
Caspase recruitment domain
- CpG:
-
Cytosine–phosphate–guanine
- DC:
-
Dendritic cells
- ECM:
-
Extracellular matrix
- GvHD:
-
Graft-versus-host disease
- IDO:
-
Indoleamine 2,3-dioxygenase
- MDP:
-
Muramyl dipeptide
- MSC:
-
Mesenchymal stromal cells
- NALP3:
-
NACHT, LRR, and PYD domains-containing protein 3
- NAD:
-
Nicotinamide adenine dinucleotide
- NLRs:
-
NOD-like receptors
- NLRPs:
-
NACHT-, LRR-, and PYD-containing proteins
- NODs:
-
Nucleotide-binding oligomerization domain
- PAMPs:
-
Pathogen-associated molecular patterns
- pDC:
-
Plasmacytoid DC
- PRR:
-
Pattern recognition receptors
- ROS:
-
Reactive oxygen species
- TLR:
-
Toll-like receptors
- Treg:
-
Regulatory T-cells
- UA:
-
Uric acid
References
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107
Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2:409–430. doi:10.1007/s11302-006-9003-5
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362:299–309
Blackburn MR, Vance CO, Morschl E, Wilson CN (2009) Adenosine receptors and inflammation. Handb Exp Pharmacol. doi:10.1007/978-3-540-89615-9_8
Blackburn MR (2003) Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci 24:66–70
Kahner BN, Shankar H, Murugappan S, Prasad GL, Kunapuli SP (2006) Nucleotide receptor signaling in platelets. J Thromb Haemost 4:2317–2326. doi:10.1111/j.1538-7836.2006.02192.x
Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A (2008) ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A 105:8067–8072
Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC (2006) Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112:358–404
Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455:808–812
Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, Hancock WW, Bach FH (1997) Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med 185:153–163
Lazarowski ER, Boucher RC, Harden TK (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64:785–795
Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR (2001) Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97:587–600
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067. doi:10.1152/physrev.00015.2002
Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176:3877–3883
Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11:201–212
Di Virgilio F, Boeynaems JM, Robson SC (2009) Extracellular nucleotides as negative modulators of immunity. Curr Opin Pharmacol 9:507–513
Praetorius HA, Leipziger J (2009) ATP release from non-excitable cells. Purinergic Signal 5:433–446
Haag F, Adriouch S, Braß A, Jung C, Möller S, Scheuplein F, Bannas P, Seman M, Koch-Nolte F (2007) Extracellular NAD and ATP: partners in immune cell modulation. Purinergic Signal 3(1–2):71–81
McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366
Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314:1792–1795. doi:10.1126/science.1132559
Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG (2010) Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal 3:ra45. doi:10.1126/scisignal.2000549
Lucattelli M, Cicko S, Muller T, Lommatzsch M, de Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Durk T, Zissel G, Sorichter S, Ferrari D, di Virgilio F, Virchow JC, Lungarella G, Idzko M (2010) P2X7 receptor signalling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol. doi:10.1165/rcmb.2010-0038OC
Lommatzsch M, Cicko S, Muller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Durk T, Zissel G, Ferrari D et al (2010) Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181:928–934. doi:10.1164/rccm.200910-1506OC
Sikora A, Liu J, Brosnan C, Buell G, Chessel I, Bloom BR (1999) Cutting edge: purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol 163:558–561
Franchi L, Eigenbrod T, Nunez G (2009) Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol 183:792–796. doi:10.4049/jimmunol.0900173
Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, Idzko M (2010) The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy 65:1545–1553. doi:10.1111/j.1398-9995.2010.02426.x
la Sala A, Sebastiani S, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G (2002) Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood 99:1715–1722
Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, Beecroft T, Davis ID, Cebon J, Maraskovsky E (2003) ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood 102:613–620. doi:10.1182/blood-2002-12-3745
Schnurr M, Then F, Galambos P, Scholz C, Siegmund B, Endres S, Eigler A (2000) Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol 165:4704–4709
Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B (2001) The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol 166:7172–7177
Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems JM (2005) Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood 106:3860–3866. doi:10.1182/blood-2005-05-1843
Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F et al (2007) Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13:913–919
Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E et al (2010) Graft-versus-host disease enhanced by extracellular adenosine triphosphate activating P2X7R. Nat Med 12:1434–1438
Baroni M, Pizzirani C, Pinotti M, Ferrari D, Adinolfi E, Calzavarini S, Caruso P, Bernardi F, Di Virgilio F (2007) Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J 21:1926–1933. doi:10.1096/fj.06-7238com
Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, Di Virgilio F (2007) Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 109:3856–3864. doi:10.1182/blood-2005-06-031377
Jurewicz M, Yang S, Augello A, Godwin JG, Moore RF, Azzi J, Fiorina P, Atkinson M, Sayegh MH, Abdi R (2010) Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 59:3139–3147. doi:10.2337/db10-0542
Joo SY, Cho KA, Jung YJ, Kim HS, Park SY, Choi YB, Hong KM, Woo SY, Seoh JY, Cho SJ, Ryu KH (2010) Mesenchymal stromal cells inhibit graft-versus-host disease of mice in a dose-dependent manner. Cytotherapy 12:361–370. doi:10.3109/14653240903502712
Ferrari D, Gulinelli S, Salvestrini V, Lucchetti G, Zini R, Manfredini R et al (2010) Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12, increases the homing capacity and the production of pro-inflammatory cytokines. Exp Hematol. doi:10.1016/j.exphem.2010.12.001
Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F (2008) Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 1:ra6. doi:10.1126/scisignal.1160583
Adriouch S, Hubert S, Pechberty S, Koch-Nolte F, Haag F, Seman M (2007) NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J Immunol 179:186–194
Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F (2003) NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 19:517–582
Shlomchik W, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG (1999) Prevention of graft-versus-host disease by inactivation of host antigen-presenting cells. Science 285:412–415
Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM (1999) Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci U S A 90:6586–6590
Anderson PO, Manzo BA, Sundstedt A, Minaee S, Symonds A, Khalid S et al (2006) Persistent antigenic stimulation alters the transcription program in T cells, resulting in antigen-specific tolerance. Eur J Immunol 36:1374–1385
Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, Koch-Nolte F, Boyer O, Seman M, Adriouch S (2010) Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2–P2X7 pathway. J Exp Med 207:2561–2568
Vogtenhuber C, Bucher C, Highfill SL, Koch LK, Goren E, Panoskaltsis-Mortari A, Taylor PA, Farrar MA, Blazar BR (2010) Constitutively active Stat5b in CD4+ T cells inhibits graft-versus-host disease (GVHD) lethality associated with increased regulatory T-cell (Treg) potency and decreased T effector cell (Teff) responses. Blood 116(3):466–474
Weber FC, Esser PR, Müller T, Ganesan J, Pellegatti P, Simon MM, Zeiser R, Idzko M, Jakob T, Martin SF (2010) Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med 207:2609–2619
Cicko S, Lucattelli M, Muller T, Lommatzsch M, De Cunto G, Cardini S et al (2010) Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol 185:688–697. doi:10.4049/jimmunol.0904042
Turner CM, Tam FW, Lai PC, Tarzi RM, Burnstock G, Pusey CD, Cook HT, Unwin RJ (2007) Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant 22:386–395. doi:10.1093/ndt/gfl589
Faas MM, van der Schaaf G, Borghuis T, Jongman RM, van Pampus MG, de Vos P, van Goor H, Bakker WW (2010) Extracellular ATP induces albuminuria in pregnant rats. Nephrol Dial Transplant 25:2468–2478. doi:10.1093/ndt/gfq095
Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM (2010) ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol 12:188–198. doi:10.1111/j.1462-5822.2009.01390.x
Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G (2006) P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol 176:2152–2160
Sharp AJ, Polak PE, Simonini V, Lin SX, Richardson JC, Bongarzone ER, Feinstein DL (2008) P2x7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J Neuroinflammation 5:33–37
Rosengren S, Hoffman HM, Bugbee W, Boyle DL (2005) Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann Rheum Dis 64:708–714
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, French LE (2007) Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol 127:1956–1963
Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA (2007) NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med 356:1216–1225
Granell M, Urbano-Ispizua A, Pons A, Aróstegui JI, Gel B, Navarro A et al (2008) Common variants in NLRP2 and NLRP3 genes are strong prognostic factors for the outcome of HLA-identical sibling allogeneic stem cell transplantation. Blood 112:4337–4342
Lee KH, Park SS, Kim I, Kim JH, Ra EK, Yoon SS, Hong YC, Park S, Kim BK (2007) P2X7 receptor polymorphism and clinical outcomes in HLA-matched sibling allogeneic hematopoietic stem cell transplantation. Haematologica 92:651–657
Schaefer L (2010) Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol 10:185–190
Schaefer L, Babelova A, Kiss E et al (2005) The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115:2223–2233
Babelova A, Moreth K, Tsalastra-Greul W et al (2009) Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem 284:24035–24048
Thomas AH, Edelman ER, Stultz CM (2007) Collagen fragments modulate innate immunity. Exp Biol Med (Maywood) 232:406–411
Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF 3rd (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276:10229–10233. doi:10.1074/jbc.M100099200
Kuhns DB, Priel DA, Gallin JI (2007) Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation 30:178–188. doi:10.1007/s10753-007-9035-1
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M (2009) Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457:102–106. doi:10.1038/nature07623
Johnson GB, Brunn GJ, Kodaira Y, Platt JL (2002) Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168:5233–5239
Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS (2010) Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol 184:2655–2662
Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E et al (2009) Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 15:774–780
Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT (2009) Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol 86:567–572. doi:10.1189/jlb.0109001
Cammalleri L, Malaguarnera M (2007) Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci 4:83–93
Shi Y, Evans JE, Rock KL (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425:516–521
Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, Stienstra R, van de Veerdonk FL, Stalenhoef AF, Giamarellos-Bourboulis EJ et al (2010) Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1beta production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum 62:3237–3248. doi:10.1002/art.27667
Kobayashi T, Kouzaki H, Kita H (2010) Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol 184:6350–6358. doi:10.4049/jimmunol.0902673
Inohara N, Ogura Y, Chen FF, Muto A, Nunez G (2001) Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem 276:2551–2554
Ferrara JL, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. Lancet 373:1550–1561. doi:10.1016/S0140-6736(09)60237-3
van Bekkum DW, Knaan S (1977) Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J Nat Cancer Inst 58:787–790
Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA et al (2010) MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59:1079–1087. doi:10.1136/gut.2009.197434
Krüger WH, Bohlius J, Cornely OA et al (2005) Antimicrobial prophylaxis in allogeneic bone marrow transplantation. Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Haematology and Oncology. Ann Oncol 16:1381–1390
Cohen J, Moore RH, Al Hashimi S, Jones L, Apperley JF, Aber VR (1987) Antibody titres to a rough-mutant strain of Escherichia coli in patients undergoing allogeneic bone-marrow transplantation: evidence of a protective effect against graft-versus-host disease. Lancet 1:8–11
Bayston K, Baumgartner JD, Clark P, Cohen J (1991) Anti-endotoxin antibody for prevention of acute GVHD. Bone Marrow Transplant 8:426–427
Calcaterra C, Sfondrini L, Rossini A, Sommariva M, Rumio C, Menard S, Balsari A (2008) Critical role of TLR9 in acute graft-versus-host disease. J Immunol 181:6132–6139
Li H, Matte-Martone C, Tan HS, Venkatesan S, McNiff J, Demetris AJ, Jain D, Lakkis F, Rothstein D, Shlomchik WD (2011) Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J Immunol 186:230–241. doi:10.4049/jimmunol.1002965
Bjorck P, Leong HX, Engleman EG (2011) Plasmacytoid dendritic cell dichotomy: identification of IFN-{alpha} producing cells as a phenotypically and functionally distinct subset. J Immunol 186:1477–1485. doi:10.4049/jimmunol.1000454
Vijay-Kumar M, Wu H, Aitken J, Kolachala VL, Neish AS, Sitaraman SV, Gewirtz AT (2007) Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis 13:856–864. doi:10.1002/ibd.20142
Hayashi T, Gray CS, Chan M, Tawatao RI, Ronacher L, McGargill MA, Datta SK, Carson DA, Corr M (2009) Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proc Natl Acad Sci U S A 106:2764–2769. doi:10.1073/pnas.0813037106
Taylor PA, Ehrhardt MJ, Lees CJ, Panoskaltsis-Mortari A, Krieg AM, Sharpe AH et al (2008) TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood 112:3508–3516. doi:10.1182/blood-2007-09-113670
Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR (2009) Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood 114:5062–5070. doi:10.1182/blood-2009-06-227587
Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, Eissner G, Schölmerich J, Andreesen R (2004) Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood 104:889–894
Hugot JP, Chamaillard M, Zouali H et al (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599–603
Ogura Y, Bonen DK et al (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411(6837):603–606
Miceli-Richard C, Lesage S et al (2001) CARD15 mutations in Blau syndrome. Nat Genet 29:19–20
Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S et al (2005) Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 105:1195–1197
Kobayashi KS, Chamaillard M et al (2005) Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731–734
Opitz B, Puschel A et al (2004) Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem 279:36426–36432
Ferwerda G, Girardin SE et al (2005) NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 1:279–285
Watanabe T, Kitani A, Murray PJ, Strober W (2004) NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 5:800–808
Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W (2006) Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25:473–485
Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M (2005) Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science 307:734–738
Penack O, Smith OM, Cunningham-Bussel A, Liu X, Rao U, Yim N et al (2009) NOD2 regulates hematopoietic cell function during graft-versus-host disease. J Exp Med 206:2101–2110. doi:10.1084/jem.20090623
Watanabe T, Asano N et al (2008) Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest 118:545–559
Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, Eissner G, Schoelmerich J, Andreesen R (2004) Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood 104(3):889–894
Holler E, Rogler G, Brenmoehl J, Hahn J, Herfarth H, Greinix H et al (2006) Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood 107:4189–4193
van der Velden WJ, Blijlevens NM, Maas FM, Schaap NP, Jansen JH, van der Reijden BA, Feuth T, Dolstra H, Donnelly JP (2009) NOD2 polymorphisms predict severe acute graft-versus-host and treatment-related mortality in T-cell-depleted haematopoietic stem cell transplantation. Bone Marrow Transplant 44:243–248. doi:10.1038/bmt.2009.21
Granell M, Urbano-Ispizua A, Arostegui JI, Fernandez-Aviles F, Martinez C, Rovira M et al (2006) Effect of NOD2/CARD15 variants in T-cell depleted allogeneic stem cell transplantation. Haematologica 9:1372–1376
van der Straaten HM, Paquay, MM, Tilanus MG, van GN, Verdonck LF, Huisman C (2011) NOD2/CARD15 variants are not a risk factor for clinical outcome after nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. doi:10.1016/j.bbmt.2010.12.709
Nguyen Y, Al-Lehibi A, Gorbe E, Li E, Haagenson M, Wang T et al (2010) Insufficient evidence for association of NOD2/CARD15 or other inflammatory bowel disease-associated markers on GVHD incidence or other adverse outcomes in T-replete, unrelated donor transplantation. Blood 115:3625–3631
Sairafi D, Uzunel M, Remberger M, Ringden O, Mattsson J (2008) No impact of NOD2/CARD15 on outcome after SCT. Bone Marrow Transplant 41:961–964
Mayor NP, Shaw BE, Hughes DA, Maldonado-Torres H, Madrigal JA, Keshav S et al (2007) Single nucleotide polymorphisms in the NOD2/CARD15 gene are associated with an increased risk of relapse and death for patients with acute leukemia after hematopoietic stem-cell transplantation with unrelated donors. J Clin Oncol 25:4262–4269
Hildebrandt GC, Granell M, Urbano-Ispizua A, Wolff D, Hertenstein B, Greinix HT et al (2008) Recipient NOD2/CARD15 variants: a novel independent risk factor for the development of bronchiolitis obliterans after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 14:67–74
Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P et al (2010) NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med 16:90–97
Holler E, Landfried K, Meier J, Hausmann M, Rogler G (2010) The role of bacteria and pattern recognition receptors in GVHD. Int J Inflamm 2010:814326
Gutierrez A, Holler E, Zapater P, Sempere L, Jover R, Perez-Mateo M et al. (2011) Antimicrobial peptide response to blood translocation of bacterial DNA in Crohn's disease is affected by NOD2/CARD15 genotype. Inflamm Bowel Dis. doi:10.1002/ibd.21537
Landfried K, Bataille F, Rogler G, Brenmoehl J, Kosovac K, Wolff D, Hilgendorf I, Hahn J, Edinger M, Hoffmann P et al (2010) Recipient NOD2/CARD15 status affects cellular infiltrates in human intestinal graft-versus-host disease. Clin Exp Immunol 159:87–92
Hill GR, Teshima T, Gerbitz A, Pan L, Cooke KR, Brinson YS, Crawford JM, Ferrara JL (1999) Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest 104:459–467
Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P et al (2009) Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med 360:2416–2425. doi:10.1056/NEJMoa0810787
Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A et al (2009) An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med 360:2426–2437. doi:10.1056/NEJMoa0807865
McCarthy PL, Williams L, Harris-Bacile M, Yen J, Przepiorka D, Ippoliti C, Champlin R, Fay J, Blosch C, Jacobs C, Anasetti C (1996) A clinical phase I/II study of recombinant human interleukin-1 receptor in glucocorticoid-resistant graft-versus-host disease. Transplantation 62:626–631
Antin JH, Weisdorf D, Neuberg D, Nicklow R, Clouthier S, Lee SJ, Alyea E et al (2002) Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood 100:3479–3482
Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J et al (2009) P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20(6):1275–1281
Matute C, Torre I, Pérez-Cerdá F, Pérez-Samartín A, Alberdi E, Etxebarria E, Arranz AM (2007) P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci 27:9525–9533
Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, Reed JC, Ting JP, Damania B (2011) Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331:330–334
Gerbitz A, Schultz M, Wilke A, Linde HJ, Schölmerich J, Andreesen R, Holler E (2004) Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood 103:4365–4367
Müller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, Idzko M (2010) Eosinophils are sensitive to ATP-induced migration and production of reactive oxygen metabolites. Allergy 65:1545–1553
Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F (1997) Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159:1451–1458
Miyazawa M, Ito Y, Kosaka N, Nukada Y, Sakaguchi H, Suzuki H, Nishiyama N (2008) Role of TNF-alpha and extracellular ATP in THP-1 cell activation following allergen exposure. J Toxicol Sci 33:71–83
Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG (2010) Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116:3475–3484. doi:10.1182/blood-2010-04-277707
Fruscione F, Scarfì S, Ferraris C, Bruzzone S, Benvenuto F, Guida L, Uccelli A, Salis A, Usai C, Jacchetti E, et al (2011) Regulation of human mesenchymal stem cell functions by an autocrine loop involving NAD(+) release and P2Y11-mediated signaling. Stem Cells Dev (in press)
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P et al (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282–286. doi:10.1038/nature08296
Thomas LM, Salter RD (2010) Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on TLR4. J Immunol 185:3740–3749. doi:10.4049/jimmunol.1001231
Hu Y, Mao K, Zeng Y, Chen S, Tao Z, Yang C, Sun S, Wu X, Meng G, Sun B (2010) Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. J Immunol 185:7699–7705
Mutini C, Falzoni S, Ferrari D, Chiozzi P, Morelli A, Baricordi OR, Collo G, Ricciardi-Castagnoli P, Di Virgilio F (1999) Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol 163:1958–1965
Lang PA, Merkler D, Funkner P, Shaabani N, Meryk A, Krings C, Barthuber C, Recher M, Bruck W, Haussinger D et al (2010) Oxidized ATP inhibits T-cell-mediated autoimmunity. Eur J Immunol 40:2401–2408. doi:10.1002/eji.200939838
Acknowledgments
We apologize to those whose work was not cited due to space limitations.
Grant support
This study was supported by the Deutsche Forschungsgemeinschaft, Germany (ID 7/4-2 to M.I., ZE 872/1-1 and Heisenberg Fellowship to R.Z. and PE 1450/1-1 to O.P.) and the Deutsche Krebshilfe, Germany (ID 108977 to O.P.).
Conflict of interests
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeiser, R., Penack, O., Holler, E. et al. Danger signals activating innate immunity in graft-versus-host disease. J Mol Med 89, 833–845 (2011). https://doi.org/10.1007/s00109-011-0767-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0767-x