Abstract
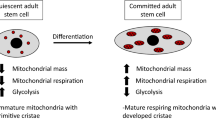
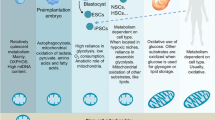
Stem cells are characterized by their multi-lineage differentiation potential (pluripotency) and their ability for self-renewal, which permits them to proliferate while avoiding lineage commitment and senescence. Recent studies demonstrate that undifferentiated, pluripotent stem cells display lower levels of mitochondrial mass and oxidative phosphorylation, and instead preferentially use non-oxidative glycolysis as a major source of energy. Hypoxia is a potent suppressor of mitochondrial oxidation and appears to promote “stemness” in adult and embryonic stem cells. This has lead to an emerging paradigm, that mitochondrial oxidative metabolism is not just an indicator of the undifferentiated state of stem cells, but may also regulate the pluripotency and self-renewal of stem cells. The identification of specific mitochondrial pathways that regulate stem cell fate may therefore enable metabolic programming and reprogramming of stem cells.

Similar content being viewed by others
References
Zhang H, Wang ZZ (2008) Mechanisms that mediate stem cell self-renewal and differentiation. J Cell Biochem 103:709–718
Orford KW, Scadden DT (2008) Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 9:115–128. doi:10.1038/nrg2269
Kim WY, Sharpless NE (2006) The regulation of INK4/ARF in cancer and aging. Cell 127:265–275. doi:10.1016/j.cell.2006.10.003
Yamanaka S (2009) A fresh look at iPS cells. Cell 137:13–17. doi:10.1016/j.cell.2009.03.034
Nishikawa S, Goldstein RA, Nierras CR (2008) The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol 9:725–729. doi:10.1038/nrm2466
Chen L, Daley GQ (2008) Molecular basis of pluripotency. Hum Mol Genet 17:R23–R27
Murry CE, Keller G (2008) Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680. doi:10.1016/j.cell.2008.02.008
Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L (2001) Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 108:407–414. doi:10.1172/JCI12131
Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L (2003) Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107:2733–2740. doi:10.1161/01.CIR.0000068356.38592.68
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM (2008) Human cardiovascular progenitor cells develop from a KDR + embryonic-stem-cell-derived population. Nature 453:524–528. doi:10.1038/nature06894
Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP (2007) Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation 116:I46–I54. doi:10.1161/CIRCULATIONAHA.106.680561
Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S (2008) Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 26:2201–2210. doi:10.1634/stemcells.2008-0428
Rehman J (2010) Feeling the elephant of cardiovascular cell therapy. Circulation 121:197–199. doi:10.1161/CIRCULATIONAHA.109.912105
Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A (2009) Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 120:408–416. doi:10.1161/CIRCULATIONAHA.109.865154
Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, Slukvin I (2009) Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27:559–567. doi:10.1634/stemcells.2008-0922
Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ (2009) Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 104:e30–e41. doi:10.1161/CIRCRESAHA.108.192237
Lensch MW, Schlaeger TM, Zon LI, Daley GQ (2007) Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell 1:253–258
Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE (2005) Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol 167:663–671.
Cho SW, Moon SH, Lee SH, Kang SW, Kim J, Lim JM, Kim HS, Kim BS, Chung HM (2007) Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation 116:2409–2419
Singh AM, Dalton S (2009) The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell 5:141–149. doi:10.1016/j.stem.2009.07.003
McBride HM, Neuspiel M, Wasiak S (2006) Mitochondria: more than just a powerhouse. Curr Biol 16:R551–R560. doi:10.1016/j.cub.2006.06.054
Zhang DX, Gutterman DD (2007) Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292:H2023–H2031. doi:10.1152/ajpheart.01283.2006
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED (2007) A mitochondria-K + channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11:37–51
Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 107:2037–2042. doi:10.1073/pnas.0914433107
Lonergan T, Brenner C, Bavister B (2006) Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol 208:149–153
Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH (2008) Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26:960–968
Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, Park do J, Park KS, Lee HK (2006) Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun 348:1472–1478
Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J (2010) The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28:721–733. doi:10.1002/stem.404
Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, Przyborski S, Lako M (2010) Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 28:661–673. doi:10.1002/stem.307
Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC (2007) Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci 120:4025–4034. doi:10.1242/jcs.016972
Facucho-Oliveira JM, St John JC (2009) The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev 5:140–158. doi:10.1007/s12015-009-9058-0
Dang CV, Le A, Gao P (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15:6479–6483. doi:10.1158/1078-0432.CCR-09-0889
Gogvadze V, Orrenius S, Zhivotovsky B (2008) Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 18:165–173. doi:10.1016/j.tcb.2008.01.006
Detmer SA, Chan DC (2007) Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8:870–879. doi:10.1038/nrm2275
Scheffler IE (2008) Mitochondria, 2nd edn. Wiley-Liss, Hoboken
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495. doi:10.1016/j.cell.2005.02.001
Denko NC (2008) Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 8:705–713
Keith B, Simon MC (2007) Hypoxia-inducible factors, stem cells, and cancer. Cell 129:465–472
Simon MC, Keith B (2008) The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 9:285–296
Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, Gassner R, Lepperdinger G (2007) Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 6:745–757
Jang YY, Sharkis SJ (2007) A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110:3056–3063
Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A 102:4783–4788
Prasad SM, Czepiel M, Cetinkaya C, Smigielska K, Weli SC, Lysdahl H, Gabrielsen A, Petersen K, Ehlers N, Fink T, Minger SL, Zachar V (2009) Continuous hypoxic culturing maintains activation of Notch and allows long-term propagation of human embryonic stem cells without spontaneous differentiation. Cell Prolif 42:63–74
Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S (2009) Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5:237–241. doi:10.1016/j.stem.2009.08.001
Varum S, Momcilovic O, Castro C, Ben-Yehudah A, Ramalho-Santos J, Navara CS (2009) Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res 3:142–156. doi:10.1016/j.scr.2009.07.002
Fulda S, Galluzzi L, Kroemer G (2010) Targeting mitochondria for cancer therapy. Nat Rev Drug Discov 9:447–464. doi:10.1038/nrd3137
Sarsour EH, Venkataraman S, Kalen AL, Oberley LW, Goswami PC (2008) Manganese superoxide dismutase activity regulates transitions between quiescent and proliferative growth. Aging Cell 7:405–417. doi:10.1111/j.1474-9726.2008.00384.x
Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A (2007) Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med 4(Suppl 1):S60–S67
Lee S, Van Remmen H, Csete M (2009) Sod2 overexpression preserves myoblast mitochondrial mass and function, but not muscle mass with aging. Aging Cell 8:296–310. doi:10.1111/j.1474-9726.2009.00477.x
Acknowledgments
This work was supported in part by NIH-K08-HL080082 (PI Jalees Rehman) and by a grant from the Heart Research Foundation (PI Jalees Rehman).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehman, J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med 88, 981–986 (2010). https://doi.org/10.1007/s00109-010-0678-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-010-0678-2