Abstract
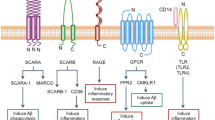
Alzheimer's disease (AD) is a progressive neurodegenerative disease. One hallmark of this disease is the continuous increase in the numbers and size of aggregating amyloid plaques. The accumulation of extracellular plaques is an immunologically interesting phenomenon since microglial cells, brain-specific macrophages, should be able to cleanse the aggregating material from the human brain. Immunotherapy targeting β-amyloid peptides in plaques with antibodies represents a promising therapy in AD. Recent progress in pattern recognition receptors of monocytes and macrophages has revealed that the sialic acid-binding, immunoglobulin-like lectin (Siglec) family of receptors is an important recognition receptor for sialylated glycoproteins and glycolipids. Interestingly, recent studies have revealed that microglial cells contain only one type of Siglec receptors, Siglec-11, which mediates immunosuppressive signals and thus inhibits the function of other microglial pattern recognition receptors, such as TLRs, NLRs, and RAGE receptors. We will review here the recent literature which clearly indicates that aggregating amyloid plaques are masked in AD by sialylated glycoproteins and gangliosides. Sialylation and glycosylation of plaques, mimicking the cell surface glycocalyx, can activate the immunosuppressive Siglec-11 receptors, as well as hiding the neuritic plaques, allowing them to evade the immune surveillance of microglial cells. This kind of immune evasion can prevent the microglial cleansing process of aggregating amyloid plaques in AD.

Similar content being viewed by others
References
Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81:741–766
Farfara D, Lifshitz V, Frenkel D (2008) Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer's disease. J Cell Mol Med 12:762–780
Armstrong RA (1998) Beta-amyloid plaques: stages in life history or independent origin. Dement Geriatr Cogn Disord 9:227–238
Guillozet AL, Weintraub S, Mash DC, Mesulam MM (2003) Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 60:729–736
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
D'Andrea MR, Cole GM, Ard MD (2004) The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol Aging 25:675–683
Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J (2004) Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol Aging 25:663–674
Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T (2009) Inflammation in Alzheimer's disease: amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Progr Neurobiol 87:181–194
Watters JJ, Schartner JM, Badie B (2005) Microglia function in brain tumors. J Neurosci Res 81:447–455
Weinbaum S, Tarbell JM, Damiano ER (2007) The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9:121–167
Ariga T, McDonald MP, Yu RK (2008) Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease—a review. J Lipid Res 49:1157–1175
Yanagisawa K (2007) Role of gangliosides in Alzheimer's disease. Biochim Biophys Acta 1768:1943–1951
Chava AK, Bandyopadhyay S, Chatterjee M, Mandal C (2004) Sialoglycans in protozoal diseases: their detection, modes of acquisition and emerging biological roles. Glycoconj J 20:199–206
Martin-Rehrmann MD, Hoe HS, Capuani EM, Rebeck GW (2005) Association of apolipoprotein J-positive β-amyloid plaques with dystrophic neurites in Alzheimer's disease brain. Neurotox Res 7:231–242
Kida E, Choi-Miura NH, Wisniewski KE (1995) Deposition of apolipoproteins E and J in senile plaques is topographically determined in both Alzheimer's disease and Down's syndrome brain. Brain Res 685:211–216
McGeer PL, Klegeris A, Walker DG, Yasuhara O, McGeer EG (1994) Pathological proteins in senile plaques. Tohoku J Exp Med 174:269–277
Wilson MR, Yerbury JJ, Poon S (2008) Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol Biosyst 4:42–52
Kalaria RN, Galloway PG, Perry G (1991) Widespread serum amyloid P immunoreactivity in cortical amyloid deposits and the neurofibrillary pathology of Alzheimer's disease and other degenerative disorders. Neuropathol Appl Neurobiol 17:189–201
Iwamoto N, Nishiyama E, Ohwada J, Arai H (1994) Demonstration of CRP immunoreactivity in brains of Alzheimer's disease: immunohistochemical study using formic acid pretreatment of tissue sections. Neurosci Lett 177:23–26
Crocker PR, Paulson JC, Varki A (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7:255–266
Von Gunten S, Bochner BS (2008) Basic and clinical immunology of Siglecs. Ann NY Acad Sci 1143:61–82
Varki A (2008) Sialic acids in human health and disease. Trends Mol Med 14:351–360
Crocker PR, Redelinghuys P (2008) Siglecs as positive and negative regulators of immune system. Biochem Soc Trans 36:1467–1471
Quarles RH (2007) Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem 100:1431–1448
McMillan SJ, Crocker PR (2008) CD33-related sialic-acid-binding immunoglobulin-like lectins in health and disease. Carbohydr Res 343:2050–2056
Chong ZZ, Maise K (2007) The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol 22:1251–1267
Rapoport E, Mikhalyov I, Zhang J, Crocker P, Bovin N (2003) Ganglioside binding pattern of CD33-related Siglecs. Bioorg Med Chem Lett 13:675–678
Angata T, Kerr C, Greaves DR, Varki NM, Crocker PR, Varki A (2002) Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem 277:24466–24474
Hayakawa T, Angata T, Lewis AL, Mikkelsen TS, Varki NM, Varki A (2005) A human-specific gene in microglia. Science 309:1693
Wang B, Miller JB, McNeil Y, McVeagh P (1998) Sialic acid concentration of brain gangliosides: variation amongst eight mammalian species. Comp Biochem Physiol 119A:435–439
Kim YJ, Kim KS, Do S, Kim CH, Kim SK, Lee YC (1997) Molecular cloning and expression of human α2, 8-sialyltransferase (hST8Sia V). Biochem Biophys Res Commun 235:327–330
Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, Jackson Roberts L 2nd, Mathews PM, Matsuoka Y, Ariga T, Yu RK, Thompson R, McDonald MP (2009) Elimination of GD3 synthase improves memory and reduces amyloid-β plaque load in transgenic mice. Neurobiol Aging (in press)
Iwamoto N, Suzuki Y, Makino Y, Haga C, Kosaka K, Iizuka R (1990) Cell membrane changes in brains manifesting senile plaques: an immunohistochemical study of GM1 membranous ganglioside. Brain Res 522:152–156
Nishinaka T, Iwata D, Shimada S, Kosaka K, Suzuki Y (1993) Anti-ganglioside GD1a monoclonal antibody recognizes senile plaques in the brains of patients with Alzheimer-type dementia. Neurosci Res 17:171–176
Mukhin DN, Chao FF, Kruth HS (1995) Glycosphingolipid accumulation in the aortic wall is another feature of human atherosclerosis. Arterioscler Thromb Vasc Biol 15:1607–1615
Nobile-Orazio E, Carpo M, Scarlato G (1994) Gangliosides. Their role in clinical neurology. Drugs 47:576–585
Svennerholm L (1994) Gangliosides—a new therapeutic agent against stroke and Alzheimer's disease. Life Sci 55:2125–2134
Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, Wilson MR (2007) The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J 21:2312–2322
Chiang KC, Goto S, Chen CL, Lin CL, Lin YC, Pan TL, Lord R, Lai CY, Tseng HP, Hsu LW, Lee TH, Yokoyama H, Kunimatsu M, Chiang YC, Hashimoto T (2000) Clusterin may be involved in rat liver allograft tolerance. Transpl Immunol 8:95–99
Nitsch RM, Hock C (2008) Targeting β-amyloid pathology in Alzheimer's disease with Aβ immunotherapy. Neurotherapeutics 5:415–420
Mohajeri MH (2007) The underestimated potential of the immune system in prevention of Alzheimer's disease pathology. Bioessays 29:927–932
Rabano A, Jimenez-Huete A, Acavedo B, Calero M, Ghiso J, Valdes I, Gavilondo J, Frangione B, Mendez E (2005) Diversity of senile plaques in Alzheimer's disease as revealed by a new monoclonal antibody that recognizes an internal sequence of the Aβ peptide. Curr Alzheimer Res 2:409–417
Morgan D (2009) The role of microglia in antibody-mediated clearance of amyloid-β from the brain. CNS Neurol Disord Drug Targets 8:7–15
Comelli EM, Head SR, Gilmartin T, Whisenant T, Haslam S, North SJ, Wong NK, Kudo T, Narimetsu H, Esko JD, Drickamer K, Dell A, Paulson JC (2006) A focused microarray approach to functional glycomics: transcriptional regulation of the glycome. Glycobiology 16:117–131
Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J (2005) Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to β-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis 18:134–142
Simard AR, Soulet D, Gowing G, Julien JP, Rivest S (2006) Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49:489–502
Acknowledgements
This study was financially supported by grants from the Academy of Finland, The Finnish Eye Foundation, and the University of Kuopio, Finland. The authors thank Dr. Ewen MacDonald for checking the language of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salminen, A., Kaarniranta, K. Siglec receptors and hiding plaques in Alzheimer's disease. J Mol Med 87, 697–701 (2009). https://doi.org/10.1007/s00109-009-0472-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-009-0472-1