Abstract
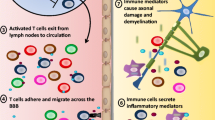
The central nervous system (CNS) is traditionally viewed as an immune privileged site in which overzealous immune cells are prevented from doing irreparable damage. It was believed that immune responses occurring within the CNS could potentially do more damage than the initial pathogenic insult itself. However, virtually every aspect of CNS tissue damage, including degeneration, tumors, infection, and of course autoimmunity, involves a significant cellular inflammatory component. While the blood–brain barrier (BBB) inhibits diffusion of hydrophilic (immune) molecules across brain capillaries, activated lymphocytes readily pass the endothelial layer of postcapillary venules without difficulty. In classic neuro-immune diseases such as multiple sclerosis or acute disseminated encephalomyelitis, it is thought that neuroantigen-reactive lymphocytes, which have escaped immune tolerance, now invade the CNS and are responsible for tissue damage, demyelination, and axonal degeneration. The developed animal model for these disorders, experimental autoimmune encephalomyelitis (EAE), reflects many aspects of the human conditions. Studies in EAE proved that auto-reactive encephalitogenic T helper (Th) cells are responsible for the onset of the disease. Th cells recognize their cognate antigen (Ag) only when presented by professional Ag-presenting cells in the context of major histocompatibility complex class II molecules. The apparent target structures of EAE immunity are myelinating oligodendrocytes, which are not capable of presenting Ag to invading encephalitogenic T cells. A compulsory third party is thus required to mediate between the attacking T cells and the myelin-expressing target. This review will discuss the recent advances in this field of research and we will discuss the journey of an auto-reactive T cell from its site of activation into perivascular spaces and further into the target tissue.


Similar content being viewed by others
References
Medawar PB (1948) Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 29:58–74
Billingham RE, Boswell T (1953) Studies on the problem of corneal homografts. Proc R Soc Lond B Biol Sci 141:392–406
Barker CF, Billingham RE (1977) Immunologically privileged sites. Adv Immunol 25:1–54
Hickey WF (2001) Basic principles of immunological surveillance of the normal central nervous system. Glia 36:118–124
Bechmann I (2005) Failed central nervous system regeneration: a downside of immune privilege? Neuromolecular Med 7:217–228
Cserr HF, Knopf PM (1992) Cervical lymphatics, the blood–brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 13:507–512
Carson MJ, Sutcliffe JG, Campbell IL (1999) Microglia stimulate naive T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res 55:127–134
Hatterer E, Davoust N, Didier-Bazes M, Vuaillat C, Malcus C, Belin M-F, Nataf S (2006) How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood 107:806–812
Karman J, Ling C, Sandor M, Fabry Z (2004) Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol 173:2353–2361
Steinman L (1996) Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85:299–302
Filippi M, Rocca MA (2003) MRI aspects of the “inflammatory phase” of multiple sclerosis. Neurol Sci 24(Suppl 5):S275–S278
Bakshi R, Hutton GJ, Miller JR, Radue EW (2004) The use of magnetic resonance imaging in the diagnosis and long-term management of multiple sclerosis. Neurology 63:S3–S11
Lin X, Blumhardt LD (2001) Inflammation and atrophy in multiple sclerosis: MRI associations with disease course. J Neurol Sci 189:99–104
Antel JP, Becher B (1998) Central nervous system–immune interactions. In: Antel J, Birnbaum G, Hartung HP (eds) Clinical Neuroimmunology. BlackwellScience Publishing House, Chapter 3, pp 26–39
Katz-Levy Y, Neville KL, Girvin AM, Vanderlugt CL, Pope JG, Tan LJ, Miller SD (1999) Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler’s virus-infected mice. J Clin Invest 104:599–610 (Comments)
McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD (2005) Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 11:335–339
Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11:328–334
Matsumoto Y, Ohmori K, Fujiwara M (1992) Immune regulation by brain cells in the central nervous system: microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions. Immunology 76:209–216
Hickey WF (1991) Migration of hematogenous cells through the blood–brain barrier and the initiation of CNS inflammation. Brain Pathol 1:97–105
Croft M, Bradley LM, Swain SL (1994) Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol 152:2675–2685
Gause WC, Lu P, Zhou XD, Chen SJ, Madden KB, Morris SC, Linsley PS, Finkelman FD, Urban JF (1996) H. polygyrus: B7-independence of the secondary type 2 response. Exp Parasitol 84:264–273
Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255–258
Friese MA, Fugger L (2005) Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain 128:1747–1763
Goverman J, Perchellet A, Huseby ES (2005) The role of CD8(+) T cells in multiple sclerosis and its animal models. Curr Drug Targets Inflamm Allergy 4:239–245
Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS (2001) Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol 166:7579–7587
Ford ML, Evavold BD (2005) Specificity, magnitude, and kinetics of MOG-specific CD8+ T cell responses during experimental autoimmune encephalomyelitis. Eur J Immunol 35:76–85
Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J (2001) A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med 194:669–676
Linker RA, Rott E, Hofstetter HH, Hanke T, Toyka KV, Gold R (2005) EAE in beta-2 microglobulin-deficient mice: axonal damage is not dependent on MHC-I restricted immune responses. Neurobiol Dis 19:218-228
Vizler C, Bercovici N, Cornet A, Cambouris C, Liblau RS (1999) Role of autoreactive CD8+ T cells in organ-specific autoimmune diseases: insight from transgenic mouse models. Immunol Rev 169:81–92
Miller JF, Morahan G (1992) Peripheral T cell tolerance. Annu Rev Immunol 10:51–69
Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR (2002) Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu Rev Neurosci 25:491–505
Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S (1997) Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med 186:307–312
Chu CQ, Wittmer S, Dalton DK (2000) Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med 192:123–128
Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H (1988) Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol 140:1506–1510
Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB (1999) IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol 163:5278–5286
Becher B, Durell BG, Noelle RJ (2002) Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest 110:493–497
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744–748
Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A (2002) IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol 169:7104–7110
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201:233–240
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132
Kwidzinski E, Mutlu LK, Kovac AD, Bunse J, Goldmann J, Mahlo J, Aktas O, Zipp F, Kamradt T, Nitsch R, Bechmann I (2003) Self-tolerance in the immune privileged CNS: lessons from the entorhinal cortex lesion model. J Neural Transm Suppl (65)29–49
Becher B, Barker PA, Owens T, Antel JP (1998) CD95-CD95L—can the brain learn from the immune system? Trends Neurosci 21:114–117
Bechmann I, Mor G, Nilsen J, Eliza M, Nitsch R, Naftolin F (1999) FasL (CD95L, Apo1L) is expressed in the normal rat and human brain: evidence for the existence of an immunological brain barrier. Glia 27:62–74
Vitkovic L, Maeda S, Sternberg E (2001) Anti-inflammatory cytokines: expression and action in the brain. Neuroimmunomodulation 9:295–312
Frohman EM, Frohman TC, Vayuvegula B, Gupta S, van den Noort S (1988) Vasoactive intestinal polypeptide inhibits the expression of the MHC class II antigens on astrocytes. J Neurol Sci 88:339–346
Hailer NP, Heppner FL, Haas D, Nitsch R (1998) Astrocytic factors deactivate antigen presenting cells that invade the central nervous system. Brain Pathol 8:459–474
Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I (2005) Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J 19:1347–1349
McGavern DB, Homann D, Oldstone MB (2002) T cells in the central nervous system: the delicate balance between viral clearance and disease. J Infect Dis 186(Suppl 2):S145–S151
Cole GA, Nathanson N, Prendergast RA (1972) Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature 238:335–337
Doherty PC, Allan JE, Lynch F, Ceredig R (1990) Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today 11:55–59
Lublin F (2005) Multiple sclerosis trial designs for the 21(st) century: building on recent lessons. J Neurol 252(Suppl 5):v46–v53
An SF, Ciardi A, Giometto B, Scaravilli T, Gray F, Scaravilli F (1996) Investigation on the expression of major histocompatibility complex class II and cytokines and detection of HIV-1 DNA within brains of asymptomatic and symptomatic HIV-1-positive patients. Acta Neuropathol (Berl) 91:494–503
Dorries R (2001) The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr Top Microbiol Immunol 253:219–245
Bechmann I, Peter S, Beyer M, Gimsa U, Nitsch R (2001) Presence of B7-2 (CD86) and lack of B7-1 (CD(80) on myelin phagocytosing MHC-II-positive rat microglia is associated with nondestructive immunity in vivo. FASEB J 15:1086–1088
Maehlen J, Olsson T, Zachau A, Klareskog L, Kristensson K (1989) Local enhancement of major histocompatibility complex (MHC) class I and II expression and cell infiltration in experimental allergic encephalomyelitis around axotomized motor neurons. J Neuroimmunol 23:125–132
McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG (1993) Microglia in degenerative neurological disease. Glia 7:84–92
O’Keefe GM, Nguyen VT, Benveniste EN (2002) Regulation and function of class II major histocompatibility complex, CD40, and B7 expression in macrophages and microglia: implications in neurological diseases. J Neurovirology 8:496–512
Perry VH (1998) A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol 90:113–121
Konno H, Yamamoto T, Suzuki h, Yamamoto H, Iwasaki Y, Ohara Y, Terunuma H, Harata N (1990) Targeting of adoptively transferred experimental allergic encephalitis lesion at the sites of wallerian degeneration. Acta Neuropathol (Berl) 80:521–526
Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Linda H, van Der Meide PH, Cullheim S, Olsson T, Piehl F (2000) Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci 20:5283–5291
Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H (2000) The neuroprotective effect of inflammation: implications for the therapy of multiple sclerosis. J Neuroimmunol 107:161–166
Schwartz M (2001) T cell mediated neuroprotection is a physiological response to central nervous system insults. J Mol Med 78:594–597
Jones TB, Ankeny DP, Guan Z, McGaughy V, Fisher LC, Basso DM, Popovich PG (2004) Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci 24:3752–3761
Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG (2002) Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci 22:2690–2700
Goebels N, Hofstetter H, Schmidt S, Brunner C, Wekerle H, Hohlfeld R (2000) Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects: epitope spreading versus clonal persistence. Brain 123(Pt 3):508–518
Meinl E, Hohlfeld R (2002) Immunopathogenesis of multiple sclerosis: MBP and beyond. Clin Exp Immunol 128:395–397
Lee SC, Raine CS (1989) Multiple sclerosis: oligodendrocytes in active lesions do not express class II major histocompatibility complex molecules. J Neuroimmunol 25:261–266
Prat A, Becher B, Antel JP (1999) Induction of B7.1 and B7.2 co-stimulatory molecules on the surface of human brain endothelial cells. J Neuroimmunol 90:24
Becher B, Prat A, Antel JP (2000) Brain–immune connection: immuno-regulatory properties of CNS-resident cells. Glia 29:293–304
Risau W, Engelhardt B, Wekerle H (1990) Immune function of the blood–brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol 110:1757–1766
Prineas JW (1979) Multiple sclerosis: presence of lymphatic capillaries and lymphoid tissue in the brain and spinal cord. Science 203:1123–1125
del Rio-Hortega P (1932) Microglia. In: Penfield W (ed) Cytology and cellular pathology of the nervous system. Hoeber, New York, pp 481–534
Schmidtmayer J, Jacobsen C, Miksch G, Sievers J (1994) Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: membrane currents. Glia 12:259–267
Streit WJ, Graeber MB (1993) Heterogeneity of microglial and perivascular cell populations: insights gained from the facial nucleus paradigm. Glia 7:68–74
Alliot F, Godin I, Pessac B (1999) Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117:145–152
Hickey WF, Vass K, Lassmann H (1992) Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol 51:246–256
Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U (2001) Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med 7:1356–1361
Becher B, Antel JP (1996) Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia 18:1–10
Sedgwick JD, Ford AL, Foulcher F, Airriess R (1998) Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J Immunol 160:5320–5330
Ulvestad E, Williams K, Bo L, Trapp B, Antel J, Mork S (1994) HLA class II molecules (HLA-DR, -DP, -DQ) on cells in the human CNS studied in situ and in vitro. Immunology 82:535–541
Aloisi F (2001) Immune function of microglia. Glia 36:165–179
Li H, Newcombe J, Groome NP, Cuzner ML (1993) Characterization and distribution of phagocytic macrophages in multiple sclerosis plaques. Neuropathol Appl Neurobiol 19:214–223
Slobodov U, Reichert F, Mirski R, Rotshenker S (2001) Distinct inflammatory stimuli induce different patterns of myelin phagocytosis and degradation in recruited macrophages. Exp Neurol 167:401–409
Williams K, Ulvestad E, Waage A, Antel JP, McLaurin J (1994) Activation of adult human derived microglia by myelin phagocytosis in vitro. J Neurosci Res 38:433–443
Olson JK, Eagar TN, Miller SD (2002) Functional activation of myelin-specific T cells by virus-induced molecular mimicry. J Immunol 169:2719–2726
Becher B, Blain M, Antel JP (2000) CD40 engagement stimulates IL-12 p70 production by human microglial cells: basis for Th1 polarization in the CNS. J Neuroimmunol 102:44–50
Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A (1987) Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol 17:1271–1278
Havenith CE, Askew D, Walker WS (1998) Mouse resident microglia: isolation and characterization of immunoregulatory properties with naive CD4+ and CD8+ T-cells. Glia 22:348–359
Krakowski ML, Owens T (1997) The central nervous system environment controls effector CD4+ T cell cytokine profile in experimental allergic encephalomyelitis. Eur J Immunol 27:2840–2847
Ford AL, Foulcher E, Lemckert FA, Sedgwick JD (1996) Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med 184:1737–1745
Juedes AE, Ruddle NH (2001) Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol 166:5168–5175
Pender MP, Rist MJ (2001) Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia 36:137–144
Howard LM, Miller SD (2001) Autoimmune intervention by CD154 blockade prevents T cell retention and effector function in the target organ. J Immunol 166:1547–1553
Weinberg AD, Wegmann KW, Funatake C, Whitham RH (1999) Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol 162:1818–1826
Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ (2001) The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J Exp Med 193:967–974
Becher B, Durell BG, Noelle RJ (2003) IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J Clin Invest 112:1186–1191
Mendel I, Shevach EM (2002) Differentiated Th1 autoreactive effector cells can induce experimental autoimmune encephalomyelitis in the absence of IL-12 and CD40/CD40L interactions. J Neuroimmunol 122:65–73
Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11:146–152
Frucht DM (2002) IL-23: a cytokine that acts on memory T cells. Sci STKE 2002:E1
Ulvestad E, Williams K, Bjerkvig R, Tiekotter K, Antel J, Matre R (1994) Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukoc Biol 56:732–740
Fontana A, Fierz W, Wekerle H (1984) Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 307:273–276
Aloisi F, Ria F, Adorini L (2000) Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today 21:141–147
De Keyser J, Zeinstra E, Frohman E (2003) Are astrocytes central players in the pathophysiology of multiple sclerosis? Arch Neurol 60:132–136
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36:180–190
Nikcevich KM, Gordon KB, Tan L, Hurst SD, Kroepfl JF, Gardinier M, Barrett TA, Miller SD (1997) IFN-gamma-activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol 158:614–621
Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175:4320–4330
Issazadeh S, Navikas V, Schaub M, Sayegh M, Khoury S (1998) Kinetics of expression of costimulatory molecules and their ligands in murine relapsing experimental autoimmune encephalomyelitis in vivo. J Immunol 161:1104–1112
Soos JM, Morrow J, Ashley TA, Szente BE, Bikoff EK, Zamvil SS (1998) Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol 161:5959–5966
Tan L, Gordon KB, Mueller JP, Matis LA, Miller SD (1998) Presentation of proteolipid protein epitopes and B7-1-dependent activation of encephalitogenic T cells by IFN-gamma-activated SJL/J astrocytes. J Immunol 160:4271–4279
Aloisi F, Ria F, Columba-Cabezas S, Hess H, Penna G, Adorini L (1999) Relative efficiency of microglia, astrocytes, dendritic cells and B cells in naive CD4+ T cell priming and Th1/Th2 cell restimulation. Eur J Immunol 29:2705–2714
Cross AH, Ku G (2000) Astrocytes and central nervous system endothelial cells do not express B7-1 (CD80) or B7-2 (CD86) immunoreactivity during experimental autoimmune encephalomyelitis. J Neuroimmunol 110:76–82
Williams KC, Dooley NP, Ulvestad E, Waage A, Blain M, Yong VW, Antel JP (1995) Antigen presentation by human fetal astrocytes with the cooperative effect of microglia or the microglial-derived cytokine IL-1. J Neurosci 15:1869–1878
Klyushnenkova EN, Vanguri P (1997) Ia expression and antigen presentation by glia: strain and cell type-specific differences among rat astrocytes and microglia. J Neuroimmunol 79:190–201
Massa PT, ter Meulen V, Fontana A (1987) Hyperinducibility of Ia antigen on astrocytes correlates with strain-specific susceptibility to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 84:4219–4223
McMenamin PG (1999) Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol 405:553–562
Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 193:905–915
Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R (2001) Turnover of rat brain perivascular cells. Exp Neurol 168:242–249
Williams K, Alvarez X, Lackner AA (2001) Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia 36:156–164
Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V (1991) Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA 88:7438–7442
Carson MJ, Reilly CR, Sutcliffe JG, Lo D (1998) Mature microglia resemble immature antigen presenting cells. Glia 22:72–85
Matyszak MK, Perry VH (1998) Bacillus Calmette–Guerin sequestered in the brain parenchyma escapes immune recognition. J Neuroimmunol 82:73–80
Hickey WF, Kimura H (1988) Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239:290–292
Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T (1998) Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol 161:3767–3775
Archambault AS, Sim J, Gimenez MA, Russell JH (2005) Defining antigen-dependent stages of T cell migration from the blood to the central nervous system parenchyma. Eur J Immunol 35:1076–1085
Murphy CA, Hoek RM, Wiekowski MT, Lira SA, Sedgwick JD (2002) Interactions between hemopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. J Immunol 169:7054–7062
Cserr HF, Knopf PM (1992) Cervical lymphatics, the blood–brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 13:507–512
de Vos AF, van Meurs M, Brok HP, Boven LA, Hintzen RQ, van der Valk P, Ravid R, Rensing S, Boon L, 't Hart BA, Laman JD (2002) Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol 169:5415–5423
Fabriek BO, Zwemmer JN, Teunissen CE, Dijkstra CD, Polman CH, Laman JD, Castelijns JA (2005) In vivo detection of myelin proteins in cervical lymph nodes of MS patients using ultrasound-guided fine-needle aspiration cytology. J Neuroimmunol 161:190–194
Lamers KJ, de Reus HP, Jongen PJ (1998) Myelin basic protein in CSF as indicator of disease activity in multiple sclerosis. Mult Scler 4:124–126
Lake J, Weller RO, Phillips MJ, Needham M (1999) Lymphocyte targeting of the brain in adoptive transfer cryolesion-EAE. J Pathol 187:259–265
Phillips MJ, Needham M, Weller RO (1997) Role of cervical lymph nodes in autoimmune encephalomyelitis in the Lewis rat. J Pathol 182:457–464
Wenkel H, Streilein JW, Young MJ (2000) Systemic immune deviation in the brain that does not depend on the integrity of the blood–brain barrier. J Immunol 164:5125–5131
Krakowski ML, Owens T (2000) Naive T lymphocytes traffic to inflamed central nervous system, but require antigen recognition for activation. Eur J Immunol 30:1002–1009
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becher, B., Bechmann, I. & Greter, M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med 84, 532–543 (2006). https://doi.org/10.1007/s00109-006-0065-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-006-0065-1