Abstract
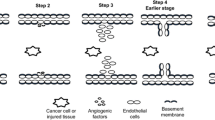
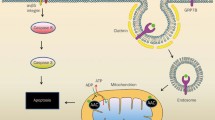
Angiogenesis, the formation of new capillary blood vessels, is a fundamental process essential for reproduction and embryonic development. It is crucial to the healing of tissue injury because it provides essential oxygen and nutrients to the healing site. Angiogenesis is also required for cancer growth and progression since tumor growth requires an increased nutrient and oxygen supply. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most widely used drugs worldwide for treating pain, arthritis, cardiovascular diseases, and more recently for colon cancer prevention. However, NSAIDs produce gastrointestinal ulcers and delay ulcer healing. Recently NSAIDs have been demonstrated to inhibit angiogenesis, but the underlying mechanisms are only beginning to be elucidated. The inhibition of angiogenesis by NSAIDs is a causal factor in the delay of ulcer healing, and it is becoming clear that this is also likely to be one of the mechanisms by which NSAIDs can reduce or prevent cancer growth. Based on the experimental data and the literature, the mechanisms by which NSAIDs inhibit angiogenesis appear to be multifactorial and likely include local changes in angiogenic growth factor expression, alteration in key regulators and mediators of vascular endothelial growth factor (VEGF), increased endothelial cell apoptosis, inhibition of endothelial cell migration, recruitment of inflammatory cells and platelets, and/or thromboxane A2 mediated effects. Some of these mechanisms include: inhibition of mitogen-activated protein (Erk2) kinase activity; suppression of cell cycle proteins; inhibition of early growth response (Egr-1) gene activation; interference with hypoxia inducible factor 1 and VEGF gene activation; increased production of the angiogenesis inhibitor, endostatin; inhibition of endothelial cell proliferation, migration, and spreading; and induction of endothelial apoptosis.





Similar content being viewed by others
Abbreviations
- bFGF :
-
Basic fibroblast growth factor
- COX :
-
Cyclooxygenase
- Egr :
-
Early growth response factor
- ERK :
-
Extracellular signal regulated kinase
- HIF :
-
Hypoxia-inducible transcription factor
- JNK :
-
Jun N-terminal kinase
- MAP :
-
Mitogen-activated protein
- NSAID :
-
Nonsteroidal anti-inflammatory drug
- PG :
-
Prostaglandin
- PGI 2 :
-
Prostacyclin
- VEGF :
-
Vascular endothelial growth factor
- VHL :
-
Von Hippel-Lindau tumor suppressor protein
References
Klagsbrun M (1991) Regulators of angiogenesis: stimulators, inhibitors and extracellular matrix. J Cell Biochem 47:190–200
Folkman J, Shin Y (1992) Angiogenesis. J Biol Chem 267:10931–10934
Folkman J, D'Amore PA (1996) Blood vessel formation: what is its molecular basis? Cell 87:1153–1155
Risau W (1997) Mechanisms of angiogenesis. Nature 386:761–764
Li J, Zhang YP, Kirsner RS (2003) Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 60:107–114
Folkman J, Browder T, Palmbald J (2001) Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost 86:23–33
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nature Review Cancer 3:401410
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9:685–693
Tarnawski A, Hollander D, Stachura J, Gergely H, Krause WJ, Sarfeh IJ (1991) Role of angiogenesis in healing of experimental gastric ulcer. In: Halter F, Garner A, Tytgat GNJ (eds) Mechanisms of peptic ulcer healing. Kluwer, Dordrecht, pp 165–171
Jones MK, Itani RM, Wang H, Tomikawa M, Sarfeh IJ, Szabo S, Tarnawski A (1999) Activation of VEGF and Ras genes in gastric mucosa during angiogenic response to ethanol injury. Am J Physiol 276:G1345–G1355
Jones MK, Mohajer B, Tomikawa M, Tarnawski AS (1999) Gastrointestinal mucosal regeneration: role of growth factors. Front Biosci 4:303–309
Constant J, Suh D, Hussain M, Hunt T (1996) Wound healing angiogenesis: the metabolic basis of repair. In: Molecular, cell, and clinical aspects of angiogenesis. Plenum, New York, pp 151–159
Ferrara N, Gerber H-P, LeCourter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Ferrara N (1999) Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56:794–814
Gerber HP, Hillan KJ, Rayan AM, Kowalski J, Kelley GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N (1999) VEGF is required for growth and survival in neonatal mice. Development 126:1149–1159
Pedram A, Razandi M, Levin ER (1998) Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem 273:26722–728
Jones MK, Sarfeh IJ, Tarnawski A (1998) Induction of in vitro angiogenesis in the endothelial-derived cell line, EA hy926, by ethanol is mediated through PKC and MAPK. Biochem Biophys Res Commun 249:118–123
Levy AP, Levy NS, Goldberg MA (1996) Post-transcriptional regulation of vascular endothelial cell growth factor by hypoxia. J Biol Chem 271:2746–2753
Gerber HJP, Condorelli F, Park J, Ferrara N (1997) Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem 272:23659–23667
Forsythe JA, Jiang B-H, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92:5510–5514
Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9:677–684
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87:1161–1169
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY (1999) Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res 58:224–237
Mitchell JA, Akarasreenont P, Thiemermann C, Flower RJ, Vane JR (1993) Selectivity of NSAIDs as inhibitors of constitutive and inducible cyclo-oxygenase. Proc Natl Acad Sci USA 90:11693–11697
Dubois RN, Abramson SB, Crofford L et al (1998) Cyclooxygenase in biology and disease. FASEB J 12:1063–1072
Wallace JL (1999) Distribution and expression of cyclooxygenase (COX) isoenzymes, their physiological roles and the categorization of nonsteroidal anti-inflammatory drugs (NSAIDs). Am J Med 107:11S-17S
Chandrasekharan NV, Dai H, Roos LT, Evanson NK, Tomsik J, Elton TS, Simmons DL (2002) COX-2 a cyclooxygenase variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proc Natl Acad Sci USA 99:13926–13931
Form DM, Auerbach R (1983) PGE2 and angiogenesis. Proc Soc Exp Biol Med 172:214–218
Spisni E, Manica F, Tomasi V (1992) Involvement of prostanoids in the regulation of angiogenesis by polypeptide growth factors. Prostaglandins Leukot Essent Fatty Acids 47:111–115
Mehrabi MR, Ekmekcioglu C, Stanek B, Thalhammer T, Tamaddon F, Pacher R, Steiner GE, Wild T, Grimm M, Spieckermann PG, Mall G, Glogar HD (2001) Angiogenesis stimulation in explanted hearts from patients pretreated with intravenous prostaglandin E1. J Heart Lung Transplant 20:465–473
Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM (1998) Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci 39:581–591
Pai R, Szabo IL, Soreghan BA, Atay S, Kawanaka H, Tarnawski AS (2001) PGE2 stimulates VEGF expression in endothelial cells via ERK2/JNK1 signaling pathways. Biochem Biophys Res Commun 286:923–928
Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, Kobayashi M, Satoh K, Narita M, Sugimoto Y, Murata T, Yoshimura H, Narumiya S, Majima M (2003) Host prostaglandin E (2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med 197:221–232
Tamura M, Sebastian S, Gurates B, Yang S, Fang Z, Bulun SE (2002) Vascular endothelial growth factor up-regulates cyclooxygenase-2 expression in human endothelial cells. J Clin Endocrinol Metab 97:3504–3507
Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, Dhiarugi V, Masisni E (2001) Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia 3:53–61
Cianchi F, Cortesini C, Bechi P, Fantappie O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R, Masini E (2001) Up-regulation of cyclooxygenase2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology 121:1339–1347
Halter F, Schamassman A, Peskar BM, Tarnawski AS (2001) Cycloxygenase-2 implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut 49:443–453
Peterson HI (1983) Effects of prostaglandin synthesis inhibitors on tumor growth and vascularization. Experimental studies in the rat. Invasion Metastasis 3:151–159
Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93:705–716
Williams CS, Mann M, DuBois RN (1999) The role of cyclooxygenases in inflammation, cancer and development. Oncogene 18:7908–7916
Sawaoka H et al (1999) Cyclooxygenase inhibitors suppress angiogenesis and reduce tumor growth in vivo. Lab Invest 79:1469–1477
Masferrer J (2001) Approach to angiogenesis inhibition based on cyclooxygenase-2. Cancer J 3:S144–S150
Kawai N, Tsujii M, Tsuji S (2002) Cyclooxygenases and colon cancer. Prostaglandins Other Lipid Mediat 69:187–196
Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC (2000) Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol 164:820–825
Husain SS, Szabo IL, Tarnawski AS (2002) NSAIDs inhibition of GI cancer growth–clinical implications and molecular mechanisms of action. Am J Gastroenterology 97:542–553
Tarnawski A, Stachura J, Douglass TG, Krause WJ, Gergely H, Sarfeh IJ (1991) Indomethacin impairs quality of experimental gastric ulcer healing: a quality histologic and ultrastructural analysis. In: Garner A, O'Brian PE (eds) Mechanism of injury, protection and repair of the upper GI tract. Wiley, Chichester, pp 521–531
Schmassmann A, Tarnawski A, Peskar BM, Varga L, Flogerzi B, Halter F (1995) Influence of acid and angiogenesis on kinetic of gastric ulcer healing in rats: interaction with indomethacin. Am J Physiol 286:G276–G285
Hudson N, Balsitis M, Everitt S, Hawkey CJ (1995) Angiogenesis in gastric ulcers: impaired in patients taking nonsteroidal anti-inflammatory drugs. Gut 37:191–194
Schmassmann A, Peskar BM, Stettler C, Netzer P, Stroff T, Flogerzi B, Halter F (1998) Effects of inhibition of prostaglandin systhase-2 in chronic gastrointestinal ulcer models in rats. Br J Pharmacol 123:795–804
Baatar D, Jones MK Jones, Pai R, Kawanaka H, Szabo IL, Moon WS, Kitano S, Tarnawski AS (2002) Selective Cox-2 blocker delays healing of experimental esophageal ulcers and inhibits ulceration-triggered c-Met/HGF receptor induction and ERK2 activation. Am J Gastroenterol 97:542–553
Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, Akamatsu T, Kasuga M (1997) Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology 112:387–397
Tarnawski A, Hollander D, Stachura J, Sarfeh IJ, Gergely H, Krause WJ (1989) Angiogenic response of gastric mucosa to ethanol injury is abolished by indomethacin (abstract). Gastroenterology 96:A505
Jones MK, Wang H, Levin E, Itani RM, Sarfeh IJ, Tarnawski AS (1999) Inhibition of angiogenesis by NSAIDs. Insight into the mechanisms and implications for cancer growth and ulcer healing. Nat Med 5:1418–1423
Pai R, Szabo IL, Kawanaka H, Soregham BA, Jones MK, Tarnawski AS (2001) Indomethacin inhibits endothelial cell proliferation by suppressing cell cycle proteins and PRB phosphorylation. A key to its anti-angiogenic action? Mol Cell Biol Res Commun 4:111–116
Bryan M, Drew GM, Houston P, Hissey P, Campbell CJ, Braddock M (2000) Tissue repair with a therapeutic transcription factor. Hum Gene Ther 11:2143–2158
Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM (2000) Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med 6:1355–1361
Stula M, Orzechowski HD, Gschwend S, Vetter R, von Harsdorf R, Dietz R, Paul M (2000) Influence of sustained mechanical stress of Egr-1 mRNA expression in cultured human endothelial cells. Mol Cell Biochem 210:101–108
Szabo IL, Pai R, Soreghan B, Jones MK, Baatar D, Kawanaka H, Tarnawski AS (2001) NSAIDs inhibit the activation of egr-1 gene in microvascular endothelial cells. A key to inhibition of angiogenesis? J Physiol (Paris) 95:379–383
Vidal F, Aragones J, Alfranca A, de Landazuri MO (2000) Up-regulation of vascular endothelial growth factor receptor Flt-1 after endothelial denudation: role of transcription factor Egr-1. Blood 95:3387–3395
Baatar D, Jones MK, Tsugawa K, Pai R, Moon WS, Koh GY, Kim I, Kitano S, Tarnawski AS (2002) Esophageal ulceration triggers expression of hypoxia-inducible factor-1 alpha and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing. Am J Pathol 161:1449–1457
Jones MK, Szabo IL, Kawanaka H, Husain SS, Tarnawski AS (2002) Von Hippel Lindau tumor suppressor and HIF-1α—new targets of NSAIDs inhibition of hypoxia-induced angiogenesis. FASEB J 16:265–266
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-α to the von Hippel-Lindau ubiquitination complex by O2-regulated prolyl hydroxylation. Science 292:468–472
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr (2001) HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464–468
Yu F, White SB, Zhao Q, Lee FS (2001) HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA 98:9630–9635
Kaelin WG Jr (2002) How oxygen makes its presence felt. Genes Dev 16:1441–1445
Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF (2002) Prostaglandin E2 induces hypoxia-inducible factor-1α stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem 227:50081–50086
Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL (2002) Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res 62:625–631
Santos AM, Sarfeh IJ, Tarnawski A, Tanoue K, Wahlstrom KJ, Knudsen KL, Irwin FL, Quina (1995) Indomethacin inhibits nitric oxide synthase mRNA and protein expression in the gastric mucosa. A key to its injurious action? Gastroenterology 108:A208
Morbidelli L, Donnini S, Ziche M (2003) Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des 9:521–530
Ma L, del Soldato P, Wallace JL (2002) Divergent effects of new cyclooxygenase inhibitors on gastric ulcer healing: shifting the angiogenic balance. Proc Natl Acad Sci USA 99:13243–13247
Ergun S, Kilic N, Wurmbach JH, Ebrahimnejad A, Fernando M, Sevinc S, Kilic E, Chalajour F, Fiedler W, Lauke H, Lamszus K, Hammerer P, Weil J, Herbst H, Folkman J (2001) Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis 4:193–206
Sales KJ, Katz AA, Howard B, Soeters RP, Millar RP, Jabbour HN, Soeters RP (2002) Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/paracrine regulation of cyclooxygenase-2, prostaglandin E receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res 62:424–432
Dormond O, Bezzi M, Mariotti A, Ruegg C (2002) Prostaglandin E2 promotes integrin αV β3-dependent endothelial cell adhesion, rac-activation, and spreading through cAMP/PKA-dependent signaling. J Biol Chem 277:45838–45846
Erickson BA, Long WE, Panesar N, Mazuski JE, Kaminski DL (1999) The effect of selective cyclooxygenase inhibitors on intestinal epithelial cell mitogenesis. J Surg Res 81:101–107
Imamine S, Akbar F, Mizukami Y, Matsui H, Onji M (2001) Apoptosis of rat gastric mucosa and of primary cultures of gastric epithelial cells by indomethacin: role of inducible nitric oxide synthase and interleukin-8. Int J Exp Pathol 82:221–229
Kelly D, Anthony A, Piasecki C, Lewin J, Pounder RE, Wakefield AJ (2000) Endothelial changes precede mucosal ulceration induced by indomethacin: an experimental study in the rat. Aliment Pharmacol Ther 14:489–496
Acknowledgements
This research was supported by the VA Medical Research Service, VA Merit Review Awards (to M.K.J. and A.S.T) and REAP (to A.S.T.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarnawski, A.S., Jones, M.K. Inhibition of angiogenesis by NSAIDs: molecular mechanisms and clinical implications. J Mol Med 81, 627–636 (2003). https://doi.org/10.1007/s00109-003-0479-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-003-0479-y