Abstract
Introduction
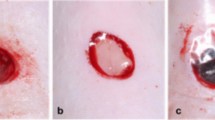
Microbial invasion in soft tissue is believed to cause infectious wounds and increase healthcare costs, anxiety, and distress. The current study was conducted to evaluate the effects of topical use of platelet-rich plasma (PRP) on infected wound-healing process in rats.
Methods
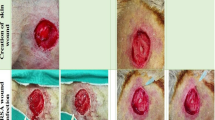
Following the induction of a circular wound, the animals were divided into three groups, including (1) standard control: infected wounds treated with mupirocin (SDCL), (2) non-infected wounds treated with PRP (PRP), and (3) infected group in which the rats were infected with Staphylococcus epidermidis and Pseudomonas aeruginosa and treated with PRP (INF + PRP). To evaluate the effects of PRP on the wound-healing rate, total bacterial count, histopathological assessment, the serum concentrations of sialic acid, C-reactive protein, haptoglobin, and fibrinogen were assessed. Additionally, IL-1β, IL-6, TNF-α, IL-3, NF-κB, iNOS, PDGF, and EGF mRNA level expressions were assessed employing the qRT-PCR method.
Results
The results indicated that topical application of PRP could significantly decrease total bacterial count, the level of C-reactive protein, and pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) compared to the SDCL group. The administration of PRP also promoted re-epithelization rate by increasing the expression of EGF mRNA level.
Conclusion
We could recommend the use of PRP for the treatment of infected wounds owing to its efficiency in decreasing colonization of tissue bacteria, tissue inflammation, and stimulating wound heal-up.




Similar content being viewed by others
References
Volodina KV, Drozdov AS, Vinogradov Vasiliy V, Vinogradov Vladimir V, Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1–2):81–94.
Petrulyte S. Advanced textile materials and biopolymers in wound management. Med Bull. 2008;55:72–7.
Cutting K. The current burden of infected wounds. Br J Health Care Manag. 2016;22:436–8.
Farahpour MR, Pirkhezr E, Ashrafian A, Sonboli A. Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed Pharmacother. 2020;1(128):110120.
Dzobo K, Turnley T, Wishart A, Rowe A, Kallmeyer K, Van Vollenstee FA, Thomford NE, Dandara C, Chopera D, Pepper MS, Parker MI. Fibroblast-derived extracellular matrix induces chondrogenic differentiation in human adipose-derived mesenchymal stromal/stem cells in vitro. Int J Mol Sci. 2016;17(8):1259.
Satish L. Chemokines as therapeutic targets to improve healing efficiency of chronic wounds. Adv Wound Care. 2015;4(11):651–9.
Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care. 2018;7(7):209–31.
Seyed Ahmadi SG, Farahpour MR, Hamishehkar H. Topical application of Cinnamon verum essential oil accelerates infected wound healing process by increasing tissue antioxidant capacity and keratin biosynthesis. Kaohsiung J Med Sci. 2019;35(11):686–94.
Ritsu M, Kawakami K, Kanno E, Tanno H, Ishii K, Imai Y, Maruyama R, Tachi M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J Dermatol Surg. 2017;21(1):14–9.
Biondo C, Mancuso G, Midiri A, Signorino G, Domina M, Cariccio VL, Mohammadi N, Venza M, Venza I, Teti G, Beninati C. The interleukin-1β/CXCL1/2/neutrophil axis mediates host protection against group B streptococcal infection. Infect Immun. 2014;82(11):4508–17.
McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, Kozma SC, Drew AF. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184(12):7219–28.
Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008.
Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R, Frei R. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. Allergy Clin Immunol. 2016;138(4):984–1010.
Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;13(9):754–64.
Futamura A, Higashiguchi T, Ito A, Kodama Y, Chihara T, Kaneko T, Tomatsu A, Shimpo K. Experimental research on stimulation of wound healing by n-3 fatty acids. Wounds. 2013;25(7):186–92.
Zuliani-Alvarez L, Midwood KS. Fibrinogen-related proteins in tissue repair: how a unique domain with a common structure controls diverse aspects of wound healing. Adv Wound Care. 2015;4(5):273–85.
MacKellar M, Vigerust DJ. Role of haptoglobin in health and disease: a focus on diabetes. Clin Diabet. 2016;34(3):148–57.
Bertaggia E, Scabia G, Dalise S, Lo Verso F, Santini F, Vitti P, Chisari C, Sandri M, Maffei M. Haptoglobin is required to prevent oxidative stress and muscle atrophy. PLoS ONE. 2014;9(6):e100745. https://doi.org/10.1371/journal.pone.0100745.
Sonar SA, Lal G. The iNOS activity during an immune response controls the CNS pathology in experimental autoimmune encephalomyelitis. Front Immunol. 2019;10:710–9.
Sadeghi-Ataabadi M, Mostafavi-Pour Z, Vojdani Z, Sani M, Latifi M, Talaei-Khozani T. Fabrication and characterization of platelet-rich plasma scaffolds for tissue engineering applications. Mater Sci Eng C. 2017;1(71):372–80.
Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24(1):16.
Farjah MH, Farahpour MR. Efficacy of topical platelet-rich plasma and chitosan co-administration on Candida albicans-infected partial thickness burn wound healing. Burns. 2020;46(8):1889–95.
Cieslik-Bielecka A, Bielecki T, Gazdzik TS, Arendt J, Król W, Szczepanski T. Autologous platelets and leukocytes can improve healing of infected high-energy soft tissue injury. Transfus Apher Sci. 2009;41(1):9–12.
Álvarez ME, López C, Giraldo CE, Samudio I, Carmona JU. In vitro bactericidal activity of equine platelet concentrates, platelet poor plasma, and plasma against methicillin-resistant Staphylococcus aureus. Arch Med Vet. 2011;43(2):155–61.
Chen J, Wan Y, Lin Y, Jiang H. The application of platelet-rich plasma for skin graft enrichment: a meta-analysis. Int Wound J. 2020;17(6):1650–8. https://doi.org/10.1111/iwj.13445.
Menchisheva Y, Mirzakulova U, Yui R. Use of platelet-rich plasma to facilitate wound healing. Int Wound J. 2019;16(2):343–53.
Xian LJ, Chowdhury SR, Saim AB, Idrus RB. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy. 2015;17(3):293–300.
Khezri K, Farahpour MR, Rad SM. Efficacy of Mentha pulegium essential oil encapsulated into nanostructured lipid carriers as an in vitro antibacterial and infected wound healing agent. Colloids Surf A. 2020;589:124414.
DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28(7):998–1009.
Oryan A, Alidadi S, Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther. 2016;16(2):213–32.
Mariani E, Filardo G, Canella V, Berlingeri A, Bielli A, Cattini L, Landini MP, Kon E, Marcacci M, Facchini A. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy. 2014;16(9):1294–304.
Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci. 2010;67(4):525–44.
Drago L, Bortolin M, Vassena C, Taschieri S, Del Fabbro M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microb. 2013;13(1):47.
Anitua E, Alonso R, Girbau C, Aguirre JJ, Muruzabal F, Orive G. Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin Exp Dermatol. 2012;37(6):652–7.
Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(01):4–15.
Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–85.
Manzuoerh R, Farahpour MR, Oryan A, Sonboli A. Effectiveness of topical administration of Anethum graveolens essential oil on MRSA-infected wounds. Biomed Pharmacother. 2019;109:1650–8.
Tong S, Zhang C, Liu J. Platelet rich plasma exhibits beneficial effects for rheumatoid arthritis mice by suppressing inflammatory factors. Mol Med Report. 2017;16:4082–8.
Kim HJ, Yeom JS, Koh YG, Yeo JE, Kang KT, Kang YM, Chang BS, Lee CK. Anti-inflammatory effect of platelet-rich plasma on nucleus pulposus cells with response of TNF-α and IL-1. J Orthop Res. 2014;32(4):551–6.
Yin W, Xu H, Sheng J, Xu Z, Xie X, Zhang C. Comparative evaluation of the effects of platelet-rich plasma formulations on extracellular matrix formation and the NF-κB signaling pathway in human articular chondrocytes. Mol Med Report. 2017;10:1–9. https://doi.org/10.3892/mmr.2017.6365.
Pereira RC, Scaranari M, Benelli R, Strada P, Reis RL, Cancedda R, Gentili C. Dual effect of platelet lysate on human articular cartilage: a maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng Part A. 2013;19:1476–88.
van Buul GM, Koevoet WL, Kops N, Bos PK, Verhaar JA, Weinans H, Bernsen MR, van Osch GJ. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39:2362–70.
Park JW, Hwang SR, Yoon IS. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules. 2017;22(8):1259.
Hudgens J, Joshua L, Grekin JA. Platelet-rich plasma activates pro-inflammatory signaling pathways and induces oxidative stress in tendon fibroblasts. Am J Sport Med. 2016;44:1931–40.
Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol. 2012;26(7):812–20.
Kim D, Kim SY, Mun SK, Rhee S, Kim BJ. Epidermal growth factor improves the migration and contractility of aged fibroblasts cultured on 3D collagen matrices. Int J Mol Med. 2015;35(4):1017–25.
Bertrand-Duchesne MP, Grenier D, Gagnon G. Epidermal growth factor released from platelet-rich plasma promotes endothelial cell proliferation in vitro. J Periodontal Res. 2010;45(1):87–93.
Hajilou H, Farahpour MR, Hamishehkar H. Polycaprolactone nanofiber coated with chitosan and Gamma oryzanol functionalized as a novel wound dressing for healing infected wounds. Int J Biol Macromol. 2020;164:2358–69.
Yamakawa S, Hayashida K. Advances in surgical applications of growth factors for wound healing. Burns Trauma. 2019;5(7):s41038–2019.
Shen C, Sun L, Zhu N, Qi F. Kindlin-1 contributes to EGF-induced re-epithelialization in skin wound healing. Int J Mol Med. 2017;39(4):949–59.
Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4(3):119–36.
Acknowledgements
This study was extracted from DVM thesis of Reza Pourkarim. The authors are grateful to Ayandeh laboratory for histological and molecular analysis. Moreover, we would like to extend gratitude to the Board of Researcheditor.ir for providing the best scientific and editorial services for scientists in Iran.
Funding
This study was the extract of a thesis research project and supported by the author’s own work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Pourkarim, R., Farahpour, M.R. & Rezaei, S.A. Comparison effects of platelet-rich plasma on healing of infected and non-infected excision wounds by the modulation of the expression of inflammatory mediators: experimental research. Eur J Trauma Emerg Surg 48, 3339–3347 (2022). https://doi.org/10.1007/s00068-022-01907-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-022-01907-0