Abstract
Background
The rates of local failure after curative radiotherapy for prostate cancer (PC) remain high despite more accurate locoregional treatments available, with one third of patients experiencing biochemical failure and clinical relapse occurring in 30–47% of cases. Today, androgen deprivation therapy (ADT) is the treatment of choice in this setting, but with not negligible toxicity and low effects on local disease. Therefore, the treatment of intraprostatic PC recurrence represents a challenge for radiation oncologists. Prostate reirradiation (Re-I) might be a therapeutic possibility. We present our series of patients treated with salvage stereotactic Re‑I for intraprostatic recurrence of PC after radical radiotherapy, with the aim of evaluating feasibility and safety of linac-based prostate Re‑I.
Materials and methods
We retrospectively evaluated toxicities and outcomes of patients who underwent salvage reirradiation using volumetric modulated arc therapy (VMAT) for intraprostatic PC recurrence. Inclusion criteria were age ≥ 18 years, histologically proven diagnosis of PC, salvage Re‑I for intraprostatic recurrence after primary radiotherapy for PC with curative intent, concurrent/adjuvant ADT with stereotactic body radiation therapy (SBRT) allowed, performance status ECOG 0–2, restaging choline/PSMA-PET/TC and prostate MRI after biochemical recurrence, and signed informed consent.
Results
From January 2019 to April 2022, 20 patients were recruited. Median follow-up was 26.7 months (range 7–50). After SBRT, no patients were lost at follow-up and all are still alive. One- and 2‑year progression free survival (PFS) was 100% and 81.5%, respectively, while 2‑year biochemical progression-free survival (bFFS) was 88.9%. Four patients (20%) experienced locoregional lymph node progression and were treated with a further course of SBRT. Prostate reirradiation allowed the ADT start to be postponed for 12–39 months. Re‑I was well tolerated by all patients and none discontinued the treatment. No cases of ≥ G3 genitourinary (GU) or gastrointestinal (GI) toxicity were reported. Seven (35%) and 2 (10%) patients experienced acute G1 and G2 GU toxicity, respectively. Late GU toxicity was recorded in 10 (50%) patients, including 8 (40%) G1 and 2 (10%) G2. ADT-related side effects were found in 7 patients (hot flashes and asthenia).
Conclusion
Linac-based SBRT is a safe technique for performing Re‑I for intraprostatic recurrence after primary curative radiotherapy for PC. Future prospective, randomized studies are desirable to better understand the effectiveness of reirradiation and the still open questions in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is one of the most common malignancies in the male population worldwide, with over 1 million new cases reported in 2018. In Italy, PC is currently the most frequent cancer diagnosis and accounts for 19% of all diagnosed cancers [1,2,3].
Primary definitive radiotherapy (RT) with or without concurrent and adjuvant androgen deprivation therapy (ADT) represents a milestone in the treatment of nonmetastatic, hormone-sensitive PC with curative intent. Despite advances in radiation treatment planning and delivery techniques, which have allowed more accurate locoregional treatments than in the past, the rates of local failure still remain high, with one third of patients experiencing biochemical failure and clinical relapse occurring in 30–47% of previously irradiated patients and in 38–54% postprostatectomy.
In this clinical scenario, the optimal management of intraprostatic recurrence after prior RT is still not standardized. Many therapeutic options are described in literature, i.e., salvage radical prostatectomy in selected cases [4], but with possible high local complication rates. Other local therapies, such as cryosurgery or high-intensity focused ultrasound (HIFU), could be considered, even if not reaching a wide consensus because of their possible adverse events, including fistula or severe rectal damage [5].
Currently, ADT is the treatment of choice in this setting, despite non-negligible systemic side effects and the probable development of castration resistance in the long term, together with little-proven benefits in terms of local control of disease [6]. Beyond these treatment modalities, reirradiation (Re-I) after local failure could be a therapeutic possibility. The critical issue of Re‑I is treatment tolerance of previously irradiated normal tissues and organs at risk (OARs) that could preclude dose delivery with curative intent [7]. However, taking into account the implementation of modern RT modalities, Re‑I has been considered as feasible for prostate cancer as for other solid tumors in clinical practice [8,9,10,11].
To date, prostate Re‑I appears to be the exclusive prerogative of very few cancer centers in the world.
The aim of the present work is to retrospectively evaluate our series of patients undergoing salvage reirradiation with a stereotactic technique for intraprostatic recurrence of PC after primary radical radiotherapy, with particular focus on toxicity outcomes and effectiveness.
Materials and methods
We retrospectively evaluated toxicities and outcomes of patients with intraprostatic recurrence following primary radical radiotherapy for hormone-sensitive prostate-confined or locally advanced PC. Intraprostatic recurrent lesions were detected by 11C-/18F-choline-positron emission tomography/computed tomography (PET/CT) or 68Ga-prostate-specific membrane antigen (PSMA)-PET/CT after evidence of biochemical relapse (defined in accordance with the Phoenix criteria: PSA ≥ PSA nadir +2 ng/mL [12, 13]). All patients underwent subsequent 1.5 T multiparametric prostate magnetic resonance imaging (mpMRI) using T2-weighted (T2W), diffusion-weighted (DWI), and dynamic contrast-enhanced (DCE) for confirmation of intraprostatic recurrence identified on functional examination (PET). In case of concordance between PET and MRI, patients were considered eligible for reirradiation. We considered prostate biopsy not mandatory if all diagnostic findings were univocal in the presence of a body of evidence (PSA kinetics, prostate MRI, and/or PET-CT findings) in favor of local recurrence.
Salvage reirradiation was performed using volumetric modulated arc therapy (VMAT). The present study received final approval from the Institutional Ethical Committee (protocol code 140/2022/OSS/IRCCSRE, approved on 27/04/2022) and was performed in accordance with the principles of Good Clinical Practice (GCP) with respect of the ICH GCP guidelines and the ethical principles contained in the Helsinki declaration and its subsequent updates. A written consent form was obtained from each enrolled patient.
Inclusion criteria
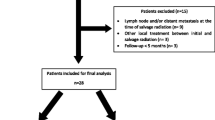
Inclusion criteria were age ≥ 18 years, histologically confirmed diagnosis of prostate cancer, patients who underwent salvage reirradiation for intraprostatic recurrence after primary radical radiotherapy for prostate cancer, concurrent/adjuvant ADT with stereotactic body radiation therapy (SBRT) was allowed, performance status ECOG 0–2, patients who underwent PET/CT and/or mpMRI for restaging after biochemical recurrence, signed informed consent.
Exclusion criteria
Exclusion criteria were performance status ECOG 3 or worse, any psychologic condition affecting the possibility to sign informed consent, patients treated with systemic therapy (chemotherapy or abiraterone/enzalutamide), the presence of extraregional metastasis at the time of relapse, patients who did not undergo restaging PET/CT and/or mpMRI after biochemical recurrence.
Radiotherapy treatment
For all patients, a simulation CT scan with 3‑mm slice thickness was performed in supine position using a Combifix immobilization system. Patients were required to have fixed (150 ml) bladder filling using a bladder catheter and an empty rectum (with enema 2 h before the procedure). Target volume delineation was performed using the ARIA® Oncology Treatment Planning System (TPS). The gross tumor volume (GTV) was outlined on the acquired CT scan. Image registration and fusion between the simulation CT scan and diagnostic mpMRI and PET/CT was performed to improve the accuracy of target volume delineation. The GTV corresponded to the visible lesion on mpMRI in case of a single intraprostatic lesion (18 patients). For bilateral recurrent lesions confirmed with MRI, the entire prostate was outlined as target volume (2 patients). A clinical target volume (CTV) was not created for stereotactic radiotherapy planning; therefore, the two volumes GTV and CTV coincided in our series. An isotropic 5–6-mm expansion was applied to the GTV/CTV to obtain the planning target volume (PTV). The outlined OARs were bladder, rectum, anterior and posterior rectal wall, femur heads, and penile bulb. The bladder catheter served as a surrogate for urethra contouring.
Radiotherapy planning was performed using VMAT with 6‑ or 10-MV flattening filter-free (FFF) beams. At least 95% of the PTV was required to receive 100% of the prescribed dose with a homogeneous distribution. Patient setup and the accuracy of target position were verified daily using cone-beam CT. A consecutive five-fraction SBRT regimen for a total target dose of 30 Gy was applied, administered every other day.
Acute toxicity was evaluated and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v.5.0 (CTCAE). The normal tissue dose constraints published by Jereczek-Fossa et al. [14] and D’Agostino et al. [15] were used for treatment planning and are summarized in Table 1.
Androgen deprivation therapy
The addition or omission of ADT was evaluated in each individual case and depended on the characteristics of the primary tumor (in particular, additional ADT was preferred for primary high-risk or locally advanced disease) and the time interval between primary treatment and local recurrence (additional ADT preferred in patients with a short-interval recurrence). ADT consisted of LH-RH analog drugs, concomitant and adjuvant to SBRT, administered for 12 months. The use of ADT was excluded in the presence of severe cardiological comorbidities.
Follow-up
The follow-up schedule consisted of clinical examination (to evaluate toxicity) and PSA detection every 3 months for the first year after Re‑I, then every 6 months. Restaging of disease with CT scan plus bone scan or functional imaging (11C-choline-PET/CT or 68Ga-PSMA PET/CT) was performed in patients with rising PSA (using the Phoenix criteria and with a focus on the PSA doubling time) and/or new-onset symptoms (urinary retention, hematuria, or bone pain refractory to pain relief).
Any symptom reported within 3 months of the end of reirradiation was considered acute toxicity; manifestation of symptoms 6 months after the end of treatment was considered late toxicity.
Definition of clinical outcome
The following parameters were used to evaluate the clinical outcomes: overall survival (OS): time between the end of reirradiation treatment and patient’s death from any cause; progression-free survival (PFS): time between the end of reirradiation treatment and the date of disease recurrence and/or progression, taking into account the sum of the events “treatment failure”; biochemical failure: defined in accordance with the Phoenix criteria (PSA ≥ PSA nadir +2 ng/mL); and finally, biochemical progression-free survival (bPFS) was defined as the time between the end of reirradiation treatment and +biochemical recurrence.
Results
From January 2019 to April 2022, 20 patients met the inclusion criteria. Median follow-up was 26.7 months (range 7–50). Median age was 78 years (range 56–82). Median time from primary RT to Re‑I was 73.8 months (range 21–146).
Characteristics of the population in primary treatment
The characteristics of the population in the study are summarized in Table 2. According to the D’Amico risk classification, 5 (25%) patients had low-risk PC, 7 (35%) intermediate-, and 8 (40%) high-risk disease at presentation. Patients were distributed in the following way regarding the ISUP grade group (GG): GG 1: 5 (25%); GG 2: 4 (20); GG 3: 8 (40); and GG 4: (15). All but one patient underwent conventionally or moderately hypofractionated radiotherapy as primary radical treatment, which was given as primary SBRT for a total dose of 36.25 Gy in 5 fractions (without pelvic irradiation). Half of the patients received pelvic irradiation, the other half were treated only to the prostate gland.
Characteristics of the population in reirradiation
Median PTV size was 13.6 cc (range 7.2–76). Reirradiation on the entire prostate was performed in 2 patients (10%) with bilateral intraglandular disease recurrence, while the target volume was represented by the single macroscopic lesion highlighted in PET/CT and mpMRI in the remaining cases (Fig. 1).
After SBRT Re‑I, no patients were lost to follow-up and all were alive at the time of the analysis. Two-year overall survival (OS) was 100%, 1‑ and 2‑year progression-free survival (PFS) were 100% and 81.5%, respectively, while 2‑year biochemical progression-free survival (bPFS) was 88.9%. Two-year local control of disease was 100%: no further intraprostatic relapse was recorded. Survival curves are showed in Fig. 2. Concurrent and adjuvant ADT with SBRT was administered in 9 patients (45%) and bPFS was evaluated by separating patients treated with and without ADT: 1–2-year bPFS without ADT was 71.4% and 28.6%, respectively, while with ADT was 69.2% and 3.8%, respectively (Fig. 3).
As regards PSA, 17 patients had a reduction in the PSA value 3 months after reirradiation, while 3 patients did not have a response in terms of PSA reduction, with an increase at the first follow-up. In all 3 cases, concomitant hormone therapy was administered, while all patients treated without concomitant hormone therapy had an initial drop in PSA.
Four patients (20%) experienced locoregional lymph node disease progression documented in PSMA-PET and were treated with a new course of SBRT. Two patients developed progression of disease 12 months after stereotactic Re‑I, the remaining two 24 months after SBRT. Biochemical failure but no pathological uptake areas on restaging PET/CT were reported in 1 (5%) patient; therefore, he continued follow-up with quarterly PSA detection. In patients who had not received concomitant hormone therapy, a postponement of ADT start by 12 to 39 months was also recorded with the use of prostate Re‑I. Moreover, all patients who received hormone therapy maintained, at the end of hormone therapy, a PSA value below the biochemical relapse value for more than 6 months from the last follow-up.
Toxicity
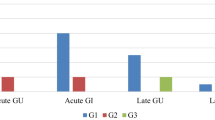
Prostate Re‑I was well tolerated by all patients any treatment interruptions were reported. Table 3 summarizes the main toxicity recorded. No ≥ G3 genitourinary (GU) or gastrointestinal (GI) acute toxicities were reported. Only 1 (5%) patient experienced G1 diarrhea. Seven (35%) and 2 (10%) patients experienced acute G1 and G2 GU toxicity, respectively. Pollakiuria, dysuria, and stranguria were the main recorded symptoms. The symptoms lasted on average 2 weeks for G2 side effects and resolved with administration of local and systemic steroid therapy. Late GU toxicity was recorded in 10 (50%) patients, including 8 (40%) G1 and 2 (10%) G2. There was no late GI toxicity of any grade. Finally, ADT-related toxicity was found with the appearance of hot flashes and asthenia.
Outcomes and toxicities
Reirradiation was well tolerated in all treated patients. Toxicities and outcomes are summarized in Table 2.
Discussion
Radiotherapy plays a crucial role in the management of PC with curative intent as well as in the salvage setting. Emerging data have established RT as a useful therapeutic option also for oligometastatic and oligorecurrent/oligoprogressive disease [16], rare histologies [17, 18], or in combination with new drugs available for hormone-sensitive and castration-resistant PC. In recent years, advances in radiation planning and delivery techniques, in particular the advent of new-generation linac with the FFF mode, have improved treatment accuracy and given rise to the adoption of ultra-hypofractionated radiation schedules in the form of SBRT [19] in different oncological settings, with acceptable toxicity [20,21,22,23,24,25].
Despite this, local failure after RT still remains a critical issue. In this setting, ADT still represents the standard therapeutic approach, with a well-known, quite scarce, and time-limited benefit, and some unavoidable consequences on the patient’s quality of life. These patients could still benefit from a local treatment with the aim of achieving local control of disease and possibly postponing the need for systemic therapies [26, 27]. Salvage prostatectomy may offer a chance of cure, although it is burdened with important sequelae such as anastomotic stricture, urinary incontinence, and rectal injury. Conversely, HIFU has been associated with lower local control rates and a higher incidence of toxicities [28]. In this scenario, prostate Re‑I represents a challenge. Some authors have speculated that disease relapse after RT may be related to radioresistant tumor cell clones; therefore, a second course of radiation may not achieve a good oncological outcome. On the other hand, local tumor control has been postulated to eliminate a possible source of metastatic spread [29], so it may be hypothesized that targeting local cancer relapse may improve the prognosis of advanced malignancies [30].
Currently, salvage prostate Re‑I is the prerogative of few centers with high-volume experience. A recent systematic review by the Reirradiation Study Group of the Italian Association of Radiation Oncology (AIRO) showed that salvage brachytherapy is the most commonly used radiation technique for post-EBRT intraprostatic tumor recurrence [31]. Brachytherapy allows delivery of high doses to the target volume with remarkable OAR sparing in view of a rapid dose fall-off outside the sources, and more and more experiences have been published on this [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51], so that it is recommended by NCCN guidelines, too. Some other reports describe prostate stereotactic Re‑I using the Cyber Knife [52,53,54,55,56,57], or external beam Re‑I with VMAT [14, 15, 58, 59]. According to the aforementioned latest systematic review of the literature [31], 18 articles are available in the current literature concerning salvage prostate Re‑I, accounting for a total of 511 patients with a median follow-up of 22 months (range 9.6–77.6). However, more recent articles have been published in the last 2 years [60,61,62,63,64,65,66,67,68].
In our series, the median follow-up was 26.7 months and the median time from primary RT to Re‑I was 73.8 months. Two-year OS and PFS were 100% and 81.5%, respectively, while 2‑year (bPFS) was 88.9%. We also found 100% local disease control and all the recruited patients were free from local failure at the time of the analysis. One- and 2‑year bPFS without ADT were 71.4% and 28.6%, respectively, while with ADT these values were 69.2% and 3.8%, respectively. We recorded only 4/20 cases of clinical progression of disease, all with nodal involvement documented by 68Ga-PSMA PET/CT and treated with a new course of ablative SBRT [16]. Unfortunately, the relatively short follow-up period and the relatively small sample size make it difficult to compare our data with those reported in literature. Nevertheless, the use of SBRT with FFF mode is relatively new in the setting of prostate Re‑I. A few studies have reported arguable survival outcomes, with 2‑year bPFS of 40–73% and 2‑year local control between 58% and 75% [31, 69].
Of note, in our series, prostate Re‑I was associated with very low toxicity rates, in line with the available literature. In fact, no ≥ G3 GU toxicity was recorded, and only 10% of the patients experienced acute G2 and late G2 GU side effects as the maximum grade of reported toxicity. Moreover, none of our patients experienced acute or late GI toxicity. Nearly one third of patients complained of ADT-related symptoms, such as hot flashes and fatigue as the most commonly described acute adverse event. Munoz et al. [31] reported a pooled result of acute ≥ G3 GU toxicity of 1.4% (95% CI 0.7–3%) and late ≥ G3 GU toxicity of 8.7% (95% CI: 5.8–13%) from 29 series, while no acute or late ≥ G3 GI toxicities occurred in the majority of the analyzed studies. In our experience, such low toxicity rates could be attributable to some of the following tools: first, SBRT performed every other day. Second, the relatively small PTV size obtained with the help of pretreatment mpMRI for target volume delineation. A pilot study by Sardaro and colleagues in 10 patients with postprostatectomy recurrent PC recently showed significantly lower mpMRI-based clinical target volumes than CT-based RT planning (p = 0.0003), with better OAR sparing and contemporary nonhomogeneous dose distribution, leading to an eventually aggressive dose escalation to the GTV [70]. The better soft tissue contrast provided by MRI and the advent of functional MR sequences may improve the definition of the prostate boundaries and pelvic OAR anatomy, the precise location of intraprostatic lesions, and thus the accuracy and safety of ablative, high-precision radiation treatments with linac, even in the setting of reirradiation [71,72,73]. Third, the use of a bladder catheter ensured high reproducibility of the treatment and excellent urethral sparing, and turned out to be fundamental to preventing obstructive and irritative urinary complications together with anti-inflammatory premedication with low doses of corticosteroids (prednisone 25 mg) concurrent to SBRT. Many authors have speculated that low toxicity rates may also depend on a long time interval between primary RT and Re‑I, while no author has demonstrated a statistical correlation between toxicity outcomes and dosimetric parameters. Some authors have correlated Re‑I of the entire prostate gland with higher toxicity rates. However, there was no increased GU or GI toxicity in the 2 patients with whole-prostate Re‑I in our series. Conversely, Zilli et al. reported severe adverse events and poor oncological outcomes using prostate Re‑I in a series of 14 patients treated with conventional or moderate hypofractionation plus brachytherapy or EBRT boost [74], and concluded that “Reirradiation on whole-gland EBRT with or without BT boost as salvage option may result in a relatively poor long-term outcome with a fairly high rate of severe side effects,” although most of the patients were treated with 3D radiotherapy in the absence of image-guided radiotherapy (IGRT).
Not less importantly, local treatments may be an attractive therapeutic strategy to postpone the need for ADT and its unavoidable long-term complications ranging from QoL impairment to metabolic changes and related systemic adverse events and, finally, the onset of tumor castration resistance [75]. The role of ADT concomitant to Re‑I is still controversial, since a clear correlation with cancer prognosis has not yet been demonstrated. Our findings showed that 45% of the patients did not receive concurrent or adjuvant ADT, while salvage prostate Re‑I allowed the start of palliative ADT to be delayed by up to 3 years. Moreover, all patients who received hormone therapy maintained, at the end of hormone therapy, a PSA below the biochemical relapse value for more than 6 months from the last follow-up, demonstrating a good biochemical response of SBRT treatment also in this patient setting.
It might be assumed a potential synergistic effect between local SBRT and systemic ADT. The su of ADT can also useful to obtain a prostate volume reduction and consequently better dose distribution [54, 76]. On contrary, the consensus advice of the Delphi group was not to administer ADT during Re‑I, probably because the advantage could be twofold, i.e., postponing an effective systemic treatment option and avoiding its potential side effects [77]. Fuller et al. [52] documented ADT-free survival (ADT-FS) as a clinical outcome, with a 5-year rate of 69%. In line with this, despite a shorter follow-up interval and a smaller sample size, a 2-year ADT-FS rate of 75% was reported by Cuccia et al. [78].
Study limitations
The main limitation of our study was its retrospective design. In addition, the small sample size significantly reduced the opportunity to identify clear risk factors and efficacy predictors related to the presented treatment approach, although few studies with a similar number of cases have been reported in literature. The relatively short follow-up time (26.7 months) must also be considered, which did not permit accurate assessment of long-term toxicity outcomes. Important variability in the characteristics of the patients during first treatment is recorded (for example, the irradiation of the pelvis, the hormonal therapy, and the presence of some patients with lymph node disease), and this could influence the outcome of the patients analyzed. Finally, ADT was administered, according to the clinical characteristics of the disease, for a duration ranging from 6 to 12 months; therefore, it was not possible to precisely define its impact on outcomes. Moreover, no patient had a rebiopsy at the time of recurrence, and therefore the diagnosis of intraprostatic recurrence was guided by PET and subsequently confirmed by MRI. The absence of a rebiopsy did not allow us to reevaluate any modification of the histological characteristics of the primary tumor at the time of recurrence. Finally, it should be noted that in our series, an intrafractional control was not used, which is often strongly suggested in this treatment setting.
Conclusion
Our experience supports the use of linac-based SBRT as a salvage reirradiation technique for intraprostatic recurrence after primary EBRT for prostate cancer as a feasible and well-tolerated treatment option with minimal toxicity. Image registration with pretreatment mpMRI and use of a bladder catheter and anti-inflammatory steroid premedication make salvage ultra-hypofractionated Re‑I with linac-FFF mode cost effective and extremely accurate. Our findings need confirmation in wider series with long-term follow-up, and randomized prospective trials are desirable.
References
Italian Medical Oncology Association (2018) Italian cancer registry by the Italian Medical Oncology Association
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rate and trends: an update. Cancer Epidemiol Biomarkers Prev 25(1):16–27. https://doi.org/10.1158/1055-9965.EPI-15-0578
Gontero P, Marra G, Alessio P, Filippini C, Oderda M, Munoz F, Linares E, Sanchez-Salas R, Challacombe B, Dasgubta P et al (2019) Salvage radical prostatectomy for recurrent prostate cancer : morbidity and functional outcomes from a large multicenter series of open versus robotic approaches. J Urol 202:725–731. https://doi.org/10.1097/JU.0000000000000327
Alongi F, De Bari B, Campostrini F, Arcangeli S, Matei DV, Lopci E, Petralia G, Bellomi M, Chiti A, Magrini SM et al (2013) Salvage therapy of intraprostatic failure after radical external-beam radiotherapy for prostate cancer: a review. Crit Rev Oncolhematol 88(3):550–563. https://doi.org/10.1016/j.critrevonc.2013.07.009
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)-Prostate Cancer, Version 2.2019—April 17, 2019. NCCN.org.
Abusaris H, Hoogeman M, Nuyttens JJ (2012) Re-Irradiation: outcome, cumulative dose and toxicity in patients retreated with stereotactic radiotherapy in the abdominal or pelvic region. Technol Cancer Res Treat 11(6):591–597. https://doi.org/10.7785/tcrt.2012.500261
Cozzi S, Augugliaro M, Ciammella P, Botti A, Trojani V, Najafi M, Blandino G, Ruggieri MP, Giaccherini L, Alì E et al (2022) The role of interstitial Brachytherapy for breast cancer treatment: an overview of indications, applications, and technical notes. Cancers 14(10):2564. https://doi.org/10.3390/cancers14102564
Cozzi S, Jamal DN, Slocker A, Laplana M, Tejedor AG, Krengli M, Guedea F, Gutierrez C (2019) Second breast-conserving therapy with interstitial brachytherapy (APBI) as a salvage treatment in ipsilateral breast tumor recurrence: a retrospective study of 40 patients. J Contemp Brachytherapy 11(2):101–107. https://doi.org/10.5114/jcb.2019.84689
Das S, Patro KC, Mukherji A (2018) Recovery and tolerance of the organs at risk during reirradiation. J Curroncol 1:23–28
Maddalo M, D’Angelo E, Fiorica F, Argenone A, Scricciolo M, Cozzi S, Nardangeli A, Dionisi F, Costantino G, Vagge S et al (2021) Thoracic re-irradiation with 3D-conformal or more advanced techniques: A systematic review of treatment safety by the Re-irradiation Study Group of the Italian Association of Radiation and Oncology AIRO. Crit Rev Oncol Hematol 167:103500. https://doi.org/10.1016/j.critrevonc.2021.103500
Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H (2001) Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol 165(4):1146–1151
Buyyounouski MK, Hanlon AL, Eisenberg DF, Horwitz EM, Feigenberg SJ, Uzzo RG, Pollack A (2005) Defining biochemical failure after radiotherapy with and without androgen deprivation for prostate cancer. Int J Radiat Oncol Biol Phys 63(5):1455–1462. https://doi.org/10.1016/j.ijrobp.2005.05.053
Jereczek-Fossa BA, Rojas DP, Zerini D, Fodor C, Viola A, Fanetti G, Volpe S, Luraschi R, Bazani A, Rondi E et al (2019) Reirradiation for isolated local recurrence of prostate cancer: Mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT). Br J Radiol 92(1094):20180494. https://doi.org/10.1259/bjr.20180494
D’Agostino GR, Di Brina L, Mancosu P, Franzese C, Iftode C, Franceschini D, Clerici E, Tozzi A, Navarria P, Scorsetti M (2019) Reirradiation of locally recurrent prostate cancer with volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 104(3):614–621. https://doi.org/10.1016/j.ijrobp.2019.02.041
Cozzi S, Botti A, Timon G, Blandino G, Najafi M, Manicone M, Bardoscia L, Ruggieri MP, Ciammella P, Iotti C (2022) Prognostic factors, efficacy, and toxicity of involved-node stereotactic body radiation therapy for lymph node oligorecurrent prostate cancer: an investigation of 117 pelvic lymph nodes. Strahlenther Onkol 198(8):700–709. https://doi.org/10.1007/s00066-021-01871-5
Cozzi S, Bardoscia L, Najafi M, Botti A, Blandino G, Augugliaro M, Manicone M, Iori F, Giaccherini L, Sardaro A et al (2022) Adenoid cystic carcinoma/basal cell carcinoma of the prostate: overview and update on rare prostate cancer subtypes. Curr Oncol 29(3):1866–1876. https://doi.org/10.3390/curroncol29030152
Bardoscia L, Triggiani L, Sandri M, Francavilla S, Borghetti P, Volta DA, Veccia A, Tomasini D, Buglione M, Valcamonico F et al (2021) Non-metastatic ductal adenocarcinoma of the prostate: pattern of care from an uro-oncology multidisciplinary group. World J Urol 39(4):1161–1170. https://doi.org/10.1007/s00345-020-03315-8
Paoletti L, Ceccarelli C, Menichelli C, Aristei C, Borghesi S, Tucci E, Bastiani P, Cozzi S (2022) Special stereotactic radiotherapy techniques: procedures and equipment for treatment simulation and dose delivery. Rep Pract Oncol Radiother 27(1):1–9. https://doi.org/10.5603/RPOR.a2021.0129
Bardoscia L, Pasinetti N, Triggiani L, Cozzi S, Sardaro A (2021) Biological bases of immune-related adverse events and potential crosslinks with Immunogenic effects of radiation. Front Pharmacol 12:746853. https://doi.org/10.3389/fphar.2021.746853
Cozzi S, Alì E, Bardoscia L, Najafi M, Botti A, Blandino G, Giaccherini L, Ruggieri MP, Augugliaro M, Iori F et al (2022) Stereotactic body radiation therapy (SBRT) for oligorecurrent/oligoprogressive mediastinal and Hilar lymph node metastasis: a systematic review. Cancers 14(11):2680. https://doi.org/10.3390/cancers14112680
Iori F, Bruni A, Cozzi S, Ciammella P, Di Pressa F, Boldrini L, Greco C, Nardone V, Salvestrini V, Desideri I et al (2022) Can radiotherapy empower the host immune system to counterattack neoplastic cells? A systematic review on tumor microenvironment radiomodulation. Curr Oncol 29(7):4612–4624. https://doi.org/10.3390/curroncol29070366
Iori F, Botti A, Ciammella P, Cozzi S, Orlandi M, Iori M, Iotti C (2022) How a very large sarcomatoid lung cancer was efficiently managed with lattice radiation therapy: a case report. Ann Palliat Med. https://doi.org/10.21037/apm-22-246
Najafi M, Jahanbakhshi A, Gomar M, Iotti C, Giaccherini L, Rezaie O, Cavallieri F, Deantonio L, Bardoscia L, Botti A et al (2022) State of the art in combination immuno/radiotherapy for brain metastases: systematic review and meta-analysis. Curr Oncol 29(5):2995–3012. https://doi.org/10.3390/curroncol29050244
Navarria P, Minniti G, Clerici E, Comito T, Cozzi S, Pinzi V, Fariselli L, Ciammella P, Scoccianti S, Borzillo V et al (2020) Brain metastases from primary colorectal cancer: is radiosurgery an effective treatment approach? Results of a multicenter study of the radiation and clinical oncology Italian association (AIRO). Br J Radiol 93(1116):20200951. https://doi.org/10.1259/bjr.20200951
Baty M, Créhange G, Pasquier D, Palard X, Deleuze A, Gnep K, Key S, Beuzit L, Castelli J, de Crevoisier R (2019) Salvage reirradiation for local prostate cancer recurrence after radiation therapy. For who? When? How? Cancer Radiother 23(6–7):541–558. https://doi.org/10.1016/j.canrad.2019.07.125
Nguyen PL, D’Amico AV, Lee AK, Suh WW (2007) Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure. Cancer 110(7):1417–1428. https://doi.org/10.1002/cncr.22941
Ingrosso G, Becherini C, Lancia A, Caini S, Ost P, Francolini G, Hoyer M, Bottero M, Bossi A, Zilli T et al (2020) Nonsurgical salvage local therapies for radiorecurrent prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol 3(2):183–197. https://doi.org/10.1016/j.euo.2018.12.011
Weckermann D, Polzer B, Ragg T, Blana A, Schlimo G, Arnholdt H, Bertz S, Harzmann R, Klein CA (2009) Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol 27(10):1549–1556. https://doi.org/10.1200/JCO.2008.17.0563
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293. https://doi.org/10.1038/nrc2621
Munoz F, Fiorica F, Caravatta L, Rosa C, Ferella L, Boldrini L, Fionda B, Alitto AR, Nardangeli A, Dionisi F et al (2021) Outcomes and toxicities of re-irradiation for prostate cancer: a systematic review on behalf of the Re-Irradiation Working Group of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Cancer Treat Rev 95:102176. https://doi.org/10.1016/j.ctrv.2021.102176
Aaronson DS, Yamasaki I, Gottschalk A, Speight J, Hsu I, Pickett B, Roach M, Shinohara K (2009) Salvage permanent perineal radioactive-seed Implantation for Treating Recurrence of Localized Prostate Adenocarcinoma After External Beam Radiotherapy. BJU Int 104(5):600–604. https://doi.org/10.1111/j.1464-410X.2009.08445.x
Allen GW, Howard AR, Jarrard DF, Ritter MA (2007) Management of prostate cancer recurrences after radiation therapy-brachytherapy as a salvage option. Cancer 110(7):1405–1416. https://doi.org/10.1002/cncr.22940
Barbera F, Triggiani L, Buglione M, Ghirardelli P, Vitali P, Caraffini B, Borghetti P, Greco D, Bardoscia L, Pasinetti N et al (2017) Salvage low dose rate brachytherapy for recurrent prostate cancer after external beam radiotherapy: results from a single institution with focus on toxicity and functional outcomes. Clin Med Insights Oncol. https://doi.org/10.1177/1179554917738765
Burri RJ, Stone NN, Unger P, Stock RG (2010) Long-term outcome and toxicity of salvage brachytherapy for local failure after initial radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 77(5):1338–1344. https://doi.org/10.1016/j.ijrobp.2009.06.061
Chen CP, Weinberg V, Shinohara K, Roach M, Nash M, Gottschalk A, Chang AJ, Hsu I (2013) Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5‑year outcomes. Int J Radiat Oncol Biol Phys 86(2):324–329. https://doi.org/10.1016/j.ijrobp.2013.01.027
Hsu CC, Hsu H, Pickett B, Crehange G, Hsu IJ, Dea R, Weinberg V, Gottschalk AR, Kurhanewicz J, Shinohara K et al (2013) Feasibility of MR Imaging/MR spectroscopy-planned focal partial salvage permanent prostate implant (PPI) for localized recurrence after initial PPI for prostate cancer. Int J Radiat Oncol Biol Phys 85(2):370–377. https://doi.org/10.1016/j.ijrobp.2012.04.028
Jo Y, Fujii T, Hara R, Yokoyama T, Miyaji Y, Yoden E, Hiratsuka J, Nagai A (2012) Salvage high-dose-rate brachytherapy for local prostate cancer recurrence after radiotherapy—preliminary results. BJU Int 109:835–839. https://doi.org/10.1111/j.1464-410X.2011.10519.x
Kollmeier MA, McBride S, Taggar A, Anderson E, Lin M, Pei X, Weiji S, Voros L, Cohen G, Yamada Y et al (2017) Salvage brachytherapy for recurrent prostate cancer after definitive radiation therapy: a comparison of low-dose-rate and high-dose-rate brachytherapy and the importance of prostate-specific antigen doubling time. Brachytherapy 16(6):1091–1098. https://doi.org/10.1016/j.brachy.2017.07.013
Koutrouvelis P, Hendricks F, Lailas N, Gil-Montero G, Sehn J, Khawand N, Bondy H, Katz S (2003) Salvage reimplantation in patient with local recurrent prostate carcinoma after brachytherapy with three dimensional computed tomography-guided permanent pararectal implant. Technol Cancer Res Treat 2(4):339–344. https://doi.org/10.1177/153303460300200409
Kukiełka AM, Hetnał M, Dąbrowski T, Walasek T, Brandys P, Nahajowski D, Kudzia R, Dybek D, Reinfuss M (2014) Salvage prostate HDR brachytherapy combined with interstitial hyperthermia for local recurrence after radiation therapy failureSalvage-HDR-Brachytherapie in Kombination mit interstitieller Hyperthermie bei Lokalrezidiv eines Prostatakarzinoms nach erfolgloser Strahlentherapie. Strahlenther Onkol 190(2):165–170. https://doi.org/10.1007/s00066-013-0486-z
Lacy JM, Wilson WA, Bole R, Chen L, Meigooni AS, Rowland RG, Clair StWH (2016) Salvage brachytherapy for biochemically recurrent prostate cancer following primary brachytherapy. Prostate Cancer 2016:1–9. https://doi.org/10.1155/2016/9561494
Lee B, Shinohara K, Weinberg V, Gottschalk AR, Pouliot J, Roach M, Hsu I (2007) Feasibility of high-dose-rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: the University of California-San Francisco Experience. Int J Radiat Oncol Biol Phys 67(4):1106–1112. https://doi.org/10.1016/j.ijrobp.2006.10.012
Łyczek J, Kawczyńska MM, Garmol D, Kasprowicz A, Kulik A, Dąbkowski M, Czyżew B, Gruszczyńska E, Bijok M, Kowalik L (2009) HDR brachytherapy as a solution in recurrences of locally advanced prostate cancer. J Contemp Brachytherapy 1(2):105–108
Maenhout M, Peters M, van Vulpen M, Moerland MA, Meijer RP, van den Bosch MAAJ, Nguyen PL, Frank SJ, van der Voort van Zyp JRN (2017) Focal MRI-guided salvage high-dose-rate brachytherapy in patients with radiorecurrent prostate cancer. Technol Cancer Res Treat 16(6):1194–1201. https://doi.org/10.1177/1533034617741797
Moman MR, van der Poel HG, Battermann JJ, Moerland MA, van Vulpen M (2010) Treatment outcome and toxicity after salvage 125‑I implantation for prostate cancer recurrences after primary 125‑I implantation and external beam radiotherapy. Brachytherapy 9(2):119–125. https://doi.org/10.1016/j.brachy.2009.06.007
Peters M, Maenhout M, van der Voort van Zyp JRN, Moerland MA, Moman MR, Steuten LMG, van Deursen MJH, van Vulpen M (2014) Focal salvage iodine-125 brachytherapy for prostate cancer recurrences after primary radiotherapy: a retrospective study regarding toxicity, biochemical outcome and quality of life. Radiother Oncol 112(1):77–82. https://doi.org/10.1016/j.radonc.2014.06.013
Rose JN, Crook JM, Pickles T, Keyes M, Morris WJ (2015) Salvage low-dose-rate permanent seed brachytherapy for locally recurrent prostate cancer: association between dose and late toxicity. Brachytherapy 14(3):342–349. https://doi.org/10.1016/j.brachy.2015.01.002
Shimbo M, Inoue K, Koike Y, Katano S, Kawashima K (2013) Salvage I seed implantation for prostate cancer with postradiation local recurrence. Urol Int 90:294–300. https://doi.org/10.1159/000346322
Vargas C, Swartz D, Vashi A, Blasser M, Kasraeian A, Cesaretti J, Kiley K, Koziol J, Terk M (2014) Salvage brachytherapy for recurrent prostate cancer. Brachytherapy 13(1):53–58. https://doi.org/10.1016/j.brachy.2013.10.012
Wojcieszek P, Szlag M, Głowacki G, Cholewka A, Gawkowska-Suwińska M, Kellas-Slęczka S, Bialas B, Fijalkowski M (2016) Salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy failure. Radiother Oncol 119(3):405–410. https://doi.org/10.1016/j.radonc.2016.04.032
Fuller DB, Wurzer J, Shirazi R, Bridge SS, Law J, Mardirossian G (2015) High-dose-rate stereotactic body radiation therapy for post radiation therapy locally recurrent prostatic carcinoma: preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol 5:e615–e623. https://doi.org/10.1016/j.prro.2015.04.0094
Janoray G, Reynaud-Bougnoux A, Ruffier-Loubière A, Bernadou G, Pointreau Y, Calais G (2016) Stereotactic body re-irradiation therapy for locally recurrent prostate cancer after external-beam radiation therapy: initial report. Cancer Radiother 20(4):275–281. https://doi.org/10.1016/j.canrad.2016.03.005
Leroy T, Lacornerie T, Bogart E, Nickers P, Lartigau E, Pasquier D (2017) Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: preliminary results of the Oscar Lambret Center. Radiat Oncol 12:95. https://doi.org/10.1186/s13014-017-0833-9
Loi M, Di Cataldo V, Simontacchi G, Detti B, Bonomo P, Masi L, Desideri I, Greto D, Francolini G, Carfora V et al (2018) Robotic stereotactic retreatment for biochemical control in previously irradiated patients affected by recurrent prostate cancer. Clin Oncol 30(2):93–100. https://doi.org/10.1016/j.clon.2017.11.007
Miszczyk L, Stąpor-Fudzinska M, Miszczyk M, Maciejewski B, Tukiendorf A (2018) Salvage cyberknife-based reirradiation of patients with recurrent prostate cancer: the single-center experience. Technol Cancer Res Treat 17:1533033818785496. https://doi.org/10.1177/1533033818785496
Olivier J, Basson L, Puech P, Lacornerie T, Villers A, Wallet J, Lartigau E, Pasquier D (2019) Stereotactic re-irradiation for local recurrence in the prostatic bed after prostatectomy: preliminary results. Front Oncol 9:71. https://doi.org/10.3389/fonc.2019.00071
Detti B, Bonomo P, Masi L, Doro R, Cipressi S, Iermano C, Bonucci I, Franceschini D, Di Brina L, Baki M et al (2016) CyberKnife stereotactic radiotherapy for isolated recurrence in the prostatic bed. World J Urol 34(3):311–317. https://doi.org/10.1007/s00345-015-1613-5
Di Franco R, Borzillo V, Scipilliti E, Ametrano G, Serra M, Arrichiello C, Savino F, De Martino F, D’Alesio V, Cammarota F et al (2022) Reirradiation of locally recurrent prostate cancer with Cyberknife® system or volumetric modulated arc therapy (VMAT) and IGRT-clarity®: outcomes, toxicities and dosimetric evaluation. Cancers 14(13):3187. https://doi.org/10.3390/cancers14133187
Cuccia F, Rigo M, Fialiua V, Giaj-Levra N, Mazzola R, Nicosia L, Ricchetti F, Trapani G, De Simone A, Guerra D et al (2022) 1.5T MR-guided daily adaptive stereotactic body radiotherapy for prostate re-irradiation: a preliminary report of toxicity and clinical outcomes. Front Oncol 12:858740. https://doi.org/10.3389/fonc.2022.858740
Fracolini G, Bellini C, Di Cataldo V, Detti B, Bruni A, Alicino G, Triggiani L, La Mattina S, D’Angelillo RM, Demofonti C et al (2022) Pattern of recurrence after stereotactic radiotherapy in prostate cancer patients with nodal pelvic relapse. A multi-institutional retrospective analysis. Clin Oncol 34(1):57–62. https://doi.org/10.1016/j.clon.2021.09.014
Perennec T, Vaugier L, Toledano A, Scher N, Thomin A, Pointreau Y, Janoray G, De Crevoisier R, Supiot S (2021) Stereotactic re-irradiation for local recurrence after radical prostatectomy and radiation therapy: a retrospective multicenter study. Cancers 13(17):4339–4327. https://doi.org/10.3390/cancers13174339
Taillez A, Bimbai AM, Lacornerie T, Le Deley MC, Lartigau EF, Pasquier D (2021) Studies of intra-fraction prostate motion during stereotactic irradiation in first irradiation and re-irradiation. Front Oncol 11:690422. https://doi.org/10.3389/fonc.2021.690422
Lewin R, Amit U, Laufer M, Berger R, Dotan Z, Domachevsky L, Davidson T, Portnoy O, Tsvang L, Ben-Ayun M, Weiss I, Symon Z (2021) Salvage re-irradiation using stereotactic body radiation therapy for locally recurrent prostate cancer: the impact of castration sensitivity on treatment outcomes. Radiat Oncol 16(1):114. https://doi.org/10.1186/s13014-021-01839-w
Matrone F, Revelant A, Fanetti G, Polesel J, Chiovati P, Avanzo M, De Renzi F, Colombo CB, Arcicasa M, De Paoli A, Franchin G, Bortolus R (2021) Partial prostate re-irradiation for the treatment of isolated local recurrence of prostate cancer in patients previously treated with primary external beam radiotherapy: short-term results of a monocentric study. Neoplasma 68(1):216–226. https://doi.org/10.4149/neo_2020_200622N651
Bergamin S, Eade T, Kneebone A, Booth J, Hsiao E, Schembri GP, Szymura K, Le A, Kwong C, Brown C et al (2020) Interim results of a prospective prostate-specific membrane antigen-directed focal stereotactic reirradiation trial for locally recurrent prostate cancer. Int J Radiat Oncol Biol Phys 108(5):1172–1178. https://doi.org/10.1016/j.ijrobp.2020.07.014
Fuller D, Wurzer J, Shirazi R, Bridge S, Law J, Crabtree T, Mardirossian G (2020) Retreatment for local recurrence of prostatic carcinoma after prior therapeutic irradiation: efficacy and toxicity of HDR-like SBRT. Int J Radiat Oncol Biol Phys 106(2):291–299. https://doi.org/10.1016/j.ijrobp.2019.10.014
Mariucci C, Ingrosso G, Bini V, Saldi S, Lupattelli M, Frattegiani A, Perrucci E, Palumbo I, Falcinelli L, Centofanti G (2020) Helical tomotherapy re-irradiation for patients affected by local radiorecurrent prostate cancer. Rep Pract Oncol Radiother 25(2):157–162. https://doi.org/10.1016/j.rpor.2020.01.005
Cuccia F, Mazzola R, Nicosia L, Giaj-Levra N, Figlia V, Ricchetti F, Rigo M, Vitale C, Corradini S, Alongi F (2020) Prostate re-irradiation: current concerns and future perspectives. Expert Rev Anticancer Ther 20(11):947–956. https://doi.org/10.1080/14737140.2020.1822742
Sardaro A, Turi B, Bardoscia L, Ferrari C, Rubibi G, Calabrese A, Ammirati F, Grillo A, Leo A, Lorusso F et al (2020) The role of multiparametric magnetic resonance in volumetric modulated arc radiation therapy planning for prostate cancer recurrence after radical prostatectomy: a pilot study. Front Oncol 10:603994. https://doi.org/10.3389/fonc.2020.603994
Ciardo D, Jereczek-Fossa BA, Petralia G, Timon G, Zerini D, Cambria R, Rondi E, Cattani F, Bazani A, al Ricottiet R (2017) Multimodal image registration for the identification of dominant intraprostatic lesion in high-precision radiotherapy treatments. Br J Radiol 90(1079):20170021. https://doi.org/10.1259/bjr.20170021
Couñago F, Sancho G, Catalá V, Hernández D, Recio M, Montemuiño S, Hernández JA, Maldonado A, Del Cerro E et al (2017) Magnetic resonance imaging for prostate cancer before radical and salvage radiotherapy: what radiation oncologists need to know. World J Clin Oncol 8(4):305–319. https://doi.org/10.5306/wjco.v8.i4.305
Steenbergen P, Haustermans K, Lerut E, Oyen R, De Wever L, Van den Bergh L, Kerkmeijer LGW, Pameijer FA, Veldhuis WB, van der Voort van Zyp JRN et al (2015) Prostate tumor delineation using multiparametric magnetic resonance imaging: Inter-observer variability and pathology validation. Radiother Oncol 115(2):186–190. https://doi.org/10.1016/j.radonc.2015.04.012
Zilli T, Benz E, Dipasquale G, Rouzaud M, Miralbell R (2016) Reirradiation of prostate cancer local failures after previous curative radiation therapy: long-term outcome and tolerance. Int J Radiat Oncol Biol Phys 96(2):318–322. https://doi.org/10.1016/j.ijrobp.2016.05.024
Harris WP, Mostaghel EA, Nelson PS, Montgomery B (2009) Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol 6(2):76–85. https://doi.org/10.1038/ncpuro1296
Triggiani L, Mazzola R, Magrini SM, Ingrosso G, Borghetti P, Trippa F, Lancia A, Detti B, Francolini G, Matrone F et al (2019) Metastasis-directed stereotactic radiotherapy for oligoprogressive castration-resistant prostate cancer: a multicenter study. World J Urol 37(12):2631–2637. https://doi.org/10.1007/s00345-019-02717-7
Kaljouw E, Pieters BR, Kovàcs G, Hoskin PJ (2016) A Delphi consensus study on salvage brachytherapy for prostate cancer relapse after radiotherapy, a Uro-GEC study. Radiother Oncol 118(1):122–130. https://doi.org/10.1016/j.radonc.2015.10.021
Cuccia F, Nicosia L, Mazzola R, Figlia V, Giaj-Levra N, Ricchetti F, Rigo M, Vitale C, Corradini S, Alongi F (2020) Linac-Based SBRT as a feasible and safe salvage retreatment approach for local recurrence in previously irradiated prostate cancer patients. Strahlenther Onkol 196(7):628–636. https://doi.org/10.1007/s00066-020-01628-6
Funding
This study was partially supported by the Italian Ministry of Health—Ricerca Corrente, Annual Program 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Cozzi, S. Finocchi Ghersi, L. Bardoscia, M. Najafi, G. Blandino, E. Alì, M. Augugliaro, F. Vigo, M.P. Ruggieri, R. Cardano, L. Giaccherini, F. Iori, A. Botti, V. Trojani, P. Ciammella, and C. Iotti declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cozzi, S., Finocchi Ghersi, S., Bardoscia, L. et al. Linac-based stereotactic salvage reirradiation for intraprostatic prostate cancer recurrence: toxicity and outcomes. Strahlenther Onkol 199, 554–564 (2023). https://doi.org/10.1007/s00066-023-02043-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02043-3