Abstract
Purpose
Magnetic resonance-guided radiotherapy (MRgRT) has recently been introduced in our institution. As MRgRT requires high patient compliance compared to conventional techniques and can be associated with prolonged treatment times, feasibility and patient tolerance were prospectively assessed using patient-reported outcome questionnaires (PRO-Q).
Materials and methods
Forty-three patients were enrolled in a prospective observational study and treated with MRgRT on a low-field hybrid Magnetic Resonance Linear Accelerator system (MR-Linac) between April 2018 and April 2019. For assistance in gated breath-hold delivery using cine-MRI, a video feedback system was installed. PRO-Qs consisted of questions on MR-related complaints and also assessed aspects of active patient participation.
Results
The most commonly treated anatomic sites were nodal metastases and liver lesions. The mean treatment time was 34 min with a mean beam-on time of 2:17 min. Gated stereotactic body radiotherapy (SBRT) was applied in 47% of all patients. Overall, patients scored MRgRT as positive or at least tolerable in the PRO‑Q. Almost two thirds of patients (65%) complained about at least one item of the PRO‑Q (score ≥4), mainly concerning coldness, paresthesia, and uncomfortable positioning. All patients reported high levels of satisfaction with their active role using the video feedback system in breath-hold delivery.
Conclusion
MRgRT was successfully implemented in our clinic and well tolerated by all patients, despite MR-related complaints and complaints about uncomfortable immobilization. Prospective clinical studies are in development for further evaluation of MRgRT and for quantification of the benefit of MR-guided on-table adaptive radiotherapy.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Image-guided radiotherapy (IGRT) allows for daily monitoring of patient and tumor positioning and, to some extent, immediate detection of alterations in tumor volume and patient anatomy [1,2,3]. Currently, image guidance is mainly based on kilovoltage or megavoltage computed tomography (CT) imaging as the standard of care, which is routinely incorporated in most modern radiotherapy units. However, onboard CT imaging only offers poor soft tissue contrast and hence primarily enables image guidance based on bony anatomy [4].
Magnetic resonance (MR) imaging, with its superior soft-tissue contrast, facilitates enhanced differentiation between cancerous and healthy tissue as well as functional assessment of treatment response [5, 6]. Given the technical challenges in integrating MR imaging (MRI) into a linear accelerator, first studies on MR-guided radiotherapy (MRgRT) focused on offline solutions and reported efficacy and feasibility for this technique [7,8,9,10].
Recently, devices integrating an MRI scanner with a treatment delivery system have become clinically available [11,12,13,14,15]. These new hybrid systems for MRgRT do not only offer three-dimensional MRI for soft tissue target and organ at risk visualization, but also allow for continuous cine-MRI before as well as during treatment [12, 13]. Cine-MRI during treatment enables advanced motion management based on real-time soft tissue anatomic feedback such as treatment beam gating [11, 16, 17]. This eliminates the need for invasive implantation of fiducial markers as well as the application of internal target volumes (ITV) in order to account for intrafractional motion, and thereby offers the potential for margin reduction and hence a lower risk of ensuing toxicity [18].
Online MRgRT was implemented at our institution in April 2018 using the MRIdian Linac® system (ViewRay Inc.; Oakwood, USA), which combines a 0.35 T MR scanner with a 6-MV linear accelerator (MR-Linac) [14, 19]. The system allows for the acquisition of three-dimensional (3D) MR scans as well as real-time tumor tracking continuously during treatment delivery by repeated fast planar cine-MRI in a sagittal plane with four frames per second [14].
All patients treated at the MR-Linac were enrolled in an observational study for evaluating feasibility as well as patient acceptance by patient-reported outcomes (PRO). In the current analysis, we describe our institutional experience with the implementation of MRgRT within a high-volume clinical center after 1 year of patient treatments, and review patient-reported outcomes.
Materials and methods
After obtaining written informed consent, all analyzed patients were included in a prospective observational clinical trial, which had been approved by the local ethics committee. For the period from April 2018 to April 2019, the trial database was interrogated for clinical information, including demographic data, dates of treatment, disease sites treated, dose and fractionation, and treatment duration.
Simulation and planning
All patients underwent MR simulation directly at the treatment machine. Thereby, not only were MR images for treatment planning generated, but tolerability of immobilization and placement of receiver coils, patient compliance to breathing instructions, and ability to perform inspiration breath-hold were also assessed. Depending on the treatment region, 3D simulation MR images were acquired either in free breathing or in inspiration breath-hold, followed by planar cine-MRI in a sagittal plane to evaluate target motion characteristics. The pulse sequence used for both was always a trueFISP sequence [14], which is the only pulse sequence currently used clinically on the system. The acquisition time of 3D simulation MR images ranged from 17 s in breath-hold to about 3 min for pelvic scans in free breathing, with an in-plane resolution of 1.5 × 1.5 mm2 and slice thickness of either 1.5 mm or 3 mm. MR simulation was carried out without administration of contrast agent. Subsequently, native CT simulation scans were performed on the same day for each patient with identical immobilization devices and simulation dummy coils, usually reproducing the same breathing state as in the MR simulation. Additional contrast-enhanced CT scans were acquired if no contraindications were present (e.g., renal dysfunction or allergies against contrast media). For treatment planning, the integrated treatment planning system (TPS) of the MRIdian Linac was used. Following simulation, MRI and CT datasets were transferred to the TPS and deformably registered using the vendor-supplied deformation algorithm, which for multimodal deformable image registration iteratively tries to minimize a dissimilarity measure computed from mutual information. When available, diagnostic MR images were additionally imported and rigidly registered with focus on the region of the GTV. Gross tumor volumes (GTV) comprised the sum of macroscopic tumor delineated in all co-registered modalities. Depending on the region to be treated, clinical target volumes (CTV) were expanded from the GTV using margins of 1 mm (for example pelvic lymph nodes) to 5 mm (for example hepatic metastases). An isotropic planning target volume (PTV) margin of 4 mm was added in order to account for technical inaccuracies.
For all patients, the simulation MRI datasets were chosen as primary image sets for treatment planning, in order to facilitate same-modality image registration during daily treatment. Electron density information for dose calculation was derived from the registered CT scans. Step-and-shoot treatment plans were generated using the dedicated TPS, where dose calculation was always performed via Monte Carlo dose calculation taking into account the static magnetic field.
Treatment
Daily image guidance was performed for each fraction by onboard 3D MRI using identical settings (field of view, duration, pulse sequence, breathing instructions) as during MR simulation. Soft tissue-based registration with the reference MR scan was applied, usually registering directly on the GTV, and the couch shifted accordingly.
Real-time MR gating was used for all patients for whom respiratory movement of the target was detected during MR simulation. All respiratory-gated treatments were performed in inspiration breath-hold. A gating target (region of interest, ROI) was defined (either the tumor/resection cavity or a surrogate, e.g., vessel, bronchus in close proximity), and the gating boundary was selected as a numerical margin added to the target ranging between 3 and 4 mm at the discretion of the treating physician. Due to uncertainties in deformable registration and image noise possibly causing errors in contour tracking [17], a small percentage of target outside of the predefined boundary without triggering a beam-off event was allowed (threshold-ROI%) [17]. Threshold-ROI% was set to 3% for most of the cases and to values up to 7% in isolated special cases. The system software automatically stopped radiation delivery when this threshold was exceeded. If intrafractional changes in target position were detected in the cine-MR, no two-dimensional table shifts were performed. Instead, the treatment was always interrupted and a repeat volumetric MRI scan was performed to allow a 3D positional correction.
During gated delivery, patients were provided with visual guidance via an in-room screen displaying the live sagittal cine-MR image with an overlay of gating target and boundary, thus enabling them to steer their repeated breath-holds to the right position. This idea was adopted from other published in-room screen solutions for MRgRT [17, 20]. Patients were able to observe this monitor during the whole treatment with the help of a mirror fixed to the bore (see Fig. 1). If necessary, patients received assisting breathing commands in order to find the optimal breath-hold. In addition to respiratory gating, the gating functionality of the system was also used for target tracking on patients in whom the target did not move with respiration, in order to ensure that no other movements happened during treatment [21].
Design of the patient-reported outcome questionnaire
Patient-reported acceptance of the whole treatment procedure was documented using an in-house developed patient-reported outcome questionnaire (PRO‑Q; see Table 1), which was completed after the first fraction, weekly during the treatment, and after the last fraction. The PRO‑Q consisted of questions regarding potential MR-related experiences and complaints (e.g., noise, bore size, fixation with coils). For patients undergoing respiratory gated treatments with audiovisual feedback, the perception of their active role was additionally evaluated. Items were scored using a five-point scale.
In addition to the experience reported by the patients, the staff on the system (therapists and physicians) were questioned about overall patient compliance for each patient after the first and last fraction. They were asked to score the overall compliance of the patient with the treatment procedure on a scale from 1 (very uncomplicated) to 10 (very complicated).
Statistical analysis
Data analysis was performed with the help of Excel 2010 (Microsoft Corporation; Redmond, USA) as well as SPSS (version 20.0; IBM, Armonk, USA). The Wilcoxon signed-rank test was applied for comparing matched samples. Significance was noted for p-values of ≤0.05.
Results
Patient and treatment characteristics
From April 2018 to April 2019, 43 patients were treated on the MRIdian Linac system, with a total of 428 fractions. Patients had a mean age of 64 years (range 32–87) at the beginning of treatment, were mainly male (58%), and had a median Karnofsky performance score of 80%. Patient characteristics and treated tumor sites are illustrated in Table 2. The most frequently treated anatomic sites were abdominal nodal metastases and liver lesions. MRgRT was selected for these patients due to a variety of reasons, including superior soft tissue contrast or gated dose delivery and mostly for a combination of these factors. For five patients with bony lesions, a benefit of soft tissue contrast was assumed because the lesions were only limitedly visible on CT scans and had been diagnosed with positron-emission tomography (PET) or scintigraphy before. Three patients with centrally located pulmonary lesions were also treated on the MRIdian Linac, as daily MRI facilitated better distinction of the target volumes from the mediastinum. Stereotactic body radiotherapy (SBRT) was applied in 20 patients (47%). Total applied doses ranged from 4 to 66 Gy, with single doses ranging from 2 to 15 Gy. The mean number of fractions per patient was nine (range 2–33). For another 23 patients, MRgRT was initially foreseen but could not be performed due to different reasons (see Table 3).
Gating was applied for all patients treated with SBRT (n = 20) as well as for eight additional patients. For all patients, the mean treatment time (time from start of acquisition of the first MRI sequence until completion of dose delivery) amounted to 34 min, with a mean beam-on time of 2:17 min. For patients treated with gated radiotherapy, the mean treatment time amounted to 40 min, while a shorter mean treatment time of 24 min was observed if no gating was applied. The mean duty cycle for respiratory gated treatments, defined as the net beam-on time per fraction divided by the overall time when the system was ready to beam during this fraction, was 72%. In about 15% of all fractions, cine-MR during treatment indicated a patient shift which then required repetition of the 3D MR-scan and repositioning of the patient before treatment could be resumed.
Patient-reported outcomes
Completed questionnaires were available for 34 patients. In total, patients rated MRgRT as positive or at least tolerable, with mean scores of 1.0–3.6 in the 14 main questions (see Table 4). No statistically significant changes were detected between the first fraction and the end of treatment for all assessed questions (p ≥ 0.05).
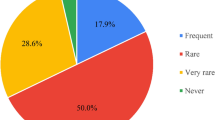
However, several patients (65%) reported some degree of potential MR-related complaints at least once (score ≥4). Patients mainly complained about the temperature in the room (24%) and of some particular body parts (27%). Furthermore, 18% of the patients experienced paresthesia during treatment and 12% rated the positioning as well as having to lie still for at least half an hour during treatment negatively (score ≥4). The size of the MRI bore and the duration of treatment were classified as at least narrow or long, respectively, by 6% of the patients. Despite the routine use of headphones, 3% of the patients scored the noise of the MRI as disturbing.
Patient experience with audiovisual feedback
The sub-section of the questionnaire related to breath-hold-gated dose delivery with audiovisual feedback was completed by 22 patients. No patient reported severe difficulties in controlling the target by holding his/her breath. Additionally, no patient answered that watching his/her tumor on the monitor during treatment was considerably disturbing. All patients seemed to appreciate their active role in controlling the duration of treatment.
Staff-rated patient compliance
The mean overall patient compliance with the treatment procedure rated by the staff was 4.1 (range 1–9) after the first fraction and 3.1 (range 1–8) after the last fraction on a scale from 1 (very uncomplicated) to 10 (very complicated) (p = 0.17).
Acute toxicity
Patients tolerated the treatment very well with only mild acute toxicity. In detail, patients reported fatigue CTCAE grade I (n = 19), nausea CTCAE grade I (n = 12), coughing CTCAE grade I (n = 7), flatulence CTCAE grade I (n = 6), diarrhea CTCAE grade I (n = 4), dyspnea CTCAE grade I (n = 2), dysphagia CTCAE grade I (n = 2), dyspepsia CTCAE grade I (n = 7), and pain in the thoracic wall CTCAE grade I (n = 2). Except for 4 patients with fatigue CTCAE grade II, no acute toxicity ≥ CTCAE grade II was detected. 11 patients did not describe any aggravation of pre-existing symptoms or the occurrence of new symptoms after RT.
Discussion
MRgRT has been successfully introduced into clinical practice at our institution. Thereby, patient-reported outcomes were an adequate tool to assess tolerability of the treatment at the MR-Linac. This is important because aside from the technical difficulties coming along with the integration of medical linear accelerators with MR scanners, the introduction of dedicated devices for MRgRT also implies new challenges for operating staff as well as patients. Patients at the MR-Linac are not only immobilized, but also need to tolerate placement of receiver coils while positioned in the bore for a longer time [22]. It is known that patients can experience claustrophobia and anxiety in an MR scanner [23, 24], and the rate usually increases with longer and narrower bore openings [23, 25]. Due to the split-magnet design [14], the MRIdian Linac has a tunnel length of about 232 cm, which is longer than at contemporary diagnostic MR scanners and could potentially contribute to anxiety [25]. While the absolute numbers of diagnostic MR scans terminated early due to claustrophobia or anxiety are generally small, in the range of 1–2% [24], early termination of fractions in radiation therapy for this reason needs to be prevented as effectively as possible.
In our cohort, all fractions could be administered safely, without patient-induced early terminations, and, as expected, without any treatment-related severe toxicities. The PRO‑Q results shown in this manuscript confirm that treatment at the MR-Linac is generally well tolerated by patients, which is in accordance with results previously published by Tetar et al. [26]. Compared to their rate of MR-related patient complaints of 29%, the value of 65% in our study is considerably higher, while, on the other hand, none of our patients reported considerable anxiety. The fact that despite a 65% complaint rate, mainly about temperature, paresthesia, and immobilization in general, the patients in our study still rated the total experience as at least tolerable, and that no patient reported considerable anxiety, might be due to the increased attention they receive from the MR-Linac staff [27, 28]. MR simulation on the MR-Linac seems to also help in the context of patient anxiety, as it allows patients to get acclimatized with the whole procedure [28]. However, thorough patient screening is still necessary in order to avoid early treatment termination due to patient noncompliance.
A limitation of this study is the relatively small number of patients. Therefore, we will continue to monitor PRO at the MR-Linac. Potentially, this information can also be used to further improve the processes in terms of patient experience [27]. Additional limitations lie in the fact that dedicated diagnostic MRI planning sequences were not available for every patient. Hence, the full potential of MRI for target delineation (particularly with the help of perfusion- or diffusion-weighted images) could not be exploited. Furthermore, diagnostic planning MRI sequences were not acquired in the treatment position, so that the co-registered images were only of limited use for target delineation, and deformable registration would have introduced additional uncertainties. Further studies involving dedicated diagnostic MRI in treatment position for target delineation as well as for follow-up imaging are therefore planned. In addition, future studies need to assess the question of which additional pulse sequences apart from the trueFISP sequence are needed for low-field MRgRT systems and how they compare to 1.5 T-systems [29].
We have shown that MR-guided respiratory gating in breath-hold is feasible and, combined with a real-time audiovisual feedback system, was very well tolerated and appreciated by patients. This confirms the positive results of Tetar et al. [26]. Cine-MR-enabled gating in breath-hold is also effective; we observed a mean gating duty cycle of 72%, similar to the range of 67% to 87% published by van Sörnsen de Koste et al. [17] using a predecessor of the MRIdian Linac system [12]. The gating duty cycle is influenced by the size of the gating boundary as well as by the threshold-ROI% value. Compared to their study, we have used slightly larger gating boundaries of up to 4 mm but, on the other hand, considerably smaller threshold-ROI% values. Apart from the paper by van Sörnsen de Koste et al. [17], a few phantom studies on the accuracy and influencing factors of MR-guided gating using low-field MRgRT systems have been published [30, 31]. However, the impact and different contributing effects still need to be studied using real patient data on a larger scale.
Besides respiratory gating, cine-MR-based structure tracking can also be used to monitor targets that do not move with respiration, for example the prostate. First published results show that this facilitates safe administration of MR-guided ultra-hypofractionated prostate treatments, potentially enabling margin reduction while eliminating the need for fiducial or transponder implantation [32].
A number of authors have reported on on-table adaption of treatment plans using MRgRT devices [11, 33,34,35,36,37] and also on assessing which patients benefit most from adaptive MRgRT [35, 38,39,40,41,42,43,44]. In the presented study, no online treatment plan adaptions were performed. Even without online adaption, MRgRT adds distinct value by better target visualization due to improved soft-tissue contrast. There might be a lot of cases where the enhanced soft tissue contrast alone improves the accuracy of radiation therapy compared to standard IGRT techniques. Noel et al. [4] have reported on physician-rated organ at risk and target visualization in onboard MR compared to onboard cone-beam CT, and visualization in MRI was rated better for 71% of all structures. There is a need for further evaluation of this aspect as well as for the opposing scenario, i.e., online adaption of treatment plans using conventional IGRT techniques.
Head-to-head comparative studies of CT-guided and MR-guided adaptive radiotherapy applying standard doses and fractionation might not be sufficient, as MR-guided adaptive radiotherapy allows for high-dose radiotherapy under circumstances in which treatment would not have been possible with conventional techniques [34]. Further well-designed clinical trials will be necessary to fully demonstrate the true potential of MR-guided adaptive radiotherapy. Radiation oncologists are now forced to reconsider the paradigms of total dose determination at the beginning of treatment and equal dose delivery during each single fraction.
Although there are many remaining questions to be answered, MR-guided adaptive radiotherapy offers the chance for tailormade, daily individualized radiotherapy in order to further reduce side-effects in cancer therapy and to improve tumor control and survival.
References
Jaffray DA (2012) Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol 9(12):688–699
Sterzing F et al (2011) Image-Guided Radiotherapy. Dtsch Arztebl Int 108(16):274–280
Dawson LA, Sharpe MB (2006) Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol 7(10):848–858
Noel CE et al (2015) Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol 54(9):1474–1482
Jones KM et al (2018) Emerging magnetic resonance imaging technologies for radiation therapy planning and response assessment. Int J Radiat Oncol Biol Phys 101(5):1046–1056
Kashani R, Olsen JR (2018) Magnetic resonance imaging for target delineation and daily treatment modification. Semin Radiat Oncol 28(3):178–184
Bostel T et al (2014) MR-guidance—a clinical study to evaluate a shuttle-based MR-linac connection to provide MR-guided radiotherapy. Radiat Oncol 9(1):12
Jaffray DA et al (2014) A facility for magnetic resonance–guided radiation therapy. Semin Radiat Oncol 24(3):193–195
Karlsson M et al (2009) Dedicated magnetic resonance imaging in the radiotherapy clinic. Int J Radiat Oncol Biol Phys 74(2):644–651
Bostel T et al (2018) Prospective feasibility analysis of a novel off-line approach for MR-guided radiotherapy. Strahlenther Onkol 194(5):425–434
Fischer-Valuck BW et al (2017) Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Advances in Radiation Oncology 2(3):485–493
Mutic S, Dempsey JF (2014) The ViewRay system: magnetic resonance–guided and controlled radiotherapy. Semin Radiat Oncol 24(3):196–199
Raaymakers BW et al (2017) First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol 62(23):L41–L50
Klüter S (2019) Technical design and concept of a 0.35 T MR-Linac. Clinical and Translational Radiation Oncology 18:98–101. https://doi.org/10.1016/j.ctro.2019.04.007
Corradini S et al (2019) MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiat Oncol 14(1):92
Bertholet J et al (2019) Real-time intrafraction motion monitoring in external beam radiotherapy. Phys Med Biol 64(15):15TR01. https://doi.org/10.1088/1361-6560/ab2ba8
van Sörnsen de Koste JR et al (2018) MR-guided gated stereotactic radiation therapy delivery for lung, adrenal, and pancreatic tumors: a geometric analysis. Int J Radiat Oncol Biol Phys 102(4):858–866
Henke LE et al (2018) Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A 4.5-year clinical experience. Clin Oncol 30(11):720–727
Liney GP et al (2018) MRI-linear accelerator radiotherapy systems. Clin Oncol 30(11):686–691
Kim J‑i et al (2017) Development of patient-controlled respiratory gating system based on visual guidance for magnetic-resonance image-guided radiation therapy. Med Phys 44(9):4838–4846
Xie Y et al (2008) Intrafractional motion of the prostate during hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys 72(1):236–246
Botman R et al (2019) The clinical introduction of MR-guided radiation therapy from a RTT perspective. Clinical and Translational Radiation Oncology 18:140–145
Dewey M, Schink T, Dewey CF (2007) Claustrophobia during magnetic resonance imaging: Cohort study in over 55,000 patients. J Magn Reson Imaging 26(5):1322–1327
Munn Z et al (2015) Claustrophobia in magnetic resonance imaging: a systematic review and meta-analysis. Radiography 21(2):e59–e63
Dantendorfer K et al (1991) Claustrophobia in MRI scanners. Lancet 338(8769):761–762
Tetar S et al (2018) Patient-reported outcome measurements on the tolerance of magnetic resonance imaging-guided radiation therapy. Cureus 10(e2236):2
Yang LY et al (2018) Patient-reported outcome use in oncology: a systematic review of the impact on patient-clinician communication. Support Care Cancer 26(1):41–60
Munn Z, Jordan Z (2011) The patient experience of high technology medical imaging: a systematic review of the qualitative evidence. Radiography 17(4):323–331
Tijssen RHN et al (2019) MRI commissioning of 1.5T MR-linac systems—a multi-institutional study. Radiother Oncol 132:114–120
Lamb JM et al (2017) Dosimetric validation of a magnetic resonance image gated radiotherapy system using a motion phantom and radiochromic film. J Appl Clin Med Phys 18(3):163–169
Green OL et al (2018) First clinical implementation of real-time, real anatomy tracking and radiation beam control. Med Phys 45(8):3728–3740. https://doi.org/10.1002/mp.13002
Bruynzeel AME et al (2019) A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: early toxicity results. Int J Radiat Oncol Biol Phys 105(5):1086–1094. https://doi.org/10.1016/j.ijrobp.2019.08.007
Lamb J et al (2017) Online adaptive radiation therapy: implementation of a new process of care. Cureus 9(8):e1618
Hunt A et al (2018) Adaptive radiotherapy enabled by MRI guidance. Clin Oncol 30(11):711–719
Boldrini L et al (2019) Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: state of the art, pearls and pitfalls. Radiat Oncol 14(1):71
Bohoudi O et al (2017) Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol 125(3):439–444
Winkel D et al (2019) Adaptive radiotherapy: the Elekta Unity MR-linac concept. Clinical and Translational Radiation Oncology 18:54–59
Bohoudi O et al (2019) Identification of patients with locally advanced pancreatic cancer benefitting from plan adaptation in MR-guided radiation therapy. Radiother Oncol 132:16–22
Henke LE et al (2018) In Silico trial of MR-guided midtreatment adaptive planning for hypofractionated stereotactic radiation therapy in centrally located thoracic tumors. Int J Radiat Oncol Biol Phys 102(4):987–995
Henke L et al (2016) Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: characterization of potential advantages. Int J Radiat Oncol Biol Phys 96(5):1078–1086
Palacios MA et al (2018) Role of daily plan adaptation in MR-guided stereotactic ablative radiation therapy for adrenal metastases. Int J Radiat Oncol Biol Phys 102(2):426–433
Werensteijn-Honingh AM et al (2019) Feasibility of stereotactic radiotherapy using a 1.5T MR-linac: Multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol 134:50–54
Henke LE et al (2019) Stereotactic MR-guided online adaptive radiation therapy (SMART) for ultracentral thorax malignancies: results of a phase 1 trial. Advances in Radiation Oncology 4(1):201–209
Rosenberg SA et al (2019) A Multi-Institutional Experience of MR-Guided Liver Stereotactic Body Radiation Therapy. Advances in Radiation Oncology 4(1):142–149
Funding
The installation of the MRIdian Linac in Heidelberg was kindly funded by the German Research Foundation DFG (project number 281540677).
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Klüter, S. Katayama, and J. Hörner-Rieber have received speaker fees and travel reimbursement from ViewRay Inc. (Oakwood, USA). J. Debus has a research agreement with ViewRay Inc. and is member of the Scientific Advisory Board of ViewRay Inc. (Oakwood, USA). C. K. Spindeldreier, S. A. Koerber, G. Major, M. Alber, and S. Akbaba declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klüter, S., Katayama, S., Spindeldreier, C.K. et al. First prospective clinical evaluation of feasibility and patient acceptance of magnetic resonance-guided radiotherapy in Germany. Strahlenther Onkol 196, 691–698 (2020). https://doi.org/10.1007/s00066-020-01578-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01578-z