Abstract
Purpose
For assessing healthy liver reactions after robotic SBRT (stereotactic body radiotherapy), we investigated early morphologic alterations on MRI (magnetic resonance imaging) with respect to patient and treatment plan parameters.
Patients and methods
MRI data at 6–17 weeks post-treatment from 22 patients with 42 liver metastases were analyzed retrospectively. Median prescription dose was 40 Gy delivered in 3–5 fractions. T2- and T1-weighted MRI were registered to the treatment plan. Absolute doses were converted to EQD2 (Equivalent dose in 2Gy fractions) with α/β-ratios of 2 and 3 Gy for healthy, and 8 Gy for modelling pre-damaged liver tissue.
Results
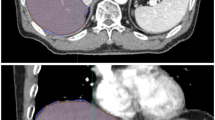
Sharply defined, centroid-shaped morphologic alterations were observed outside the high-dose volume surrounding the GTV. On T2-w MRI, hyperintensity at EQD2 isodoses of 113.3 ± 66.1 Gy2, 97.5 ± 54.7 Gy3, and 66.5 ± 32.0 Gy8 significantly depended on PTV dimension (p = 0.02) and healthy liver EQD2 (p = 0.05). On T1-w non-contrast MRI, hypointensity at EQD2 isodoses of 113.3 ± 49.3 Gy2, 97.4 ± 41.0 Gy3, and 65.7 ± 24.2 Gy8 significantly depended on prior chemotherapy (p = 0.01) and total liver volume (p = 0.05). On T1-w gadolinium-contrast delayed MRI, hypointensity at EQD2 isodoses of 90.6 ± 42.5 Gy2, 79.3 ± 35.3 Gy3, and 56.6 ± 20.9 Gy8 significantly depended on total (p = 0.04) and healthy (p = 0.01) liver EQD2.
Conclusions
Early post-treatment changes in healthy liver tissue after robotic SBRT could spatially be correlated to respective isodoses. Median nominal doses of 10.1–11.3 Gy per fraction (EQD2 79–97 Gy3) induce characteristic morphologic alterations surrounding the lesions, potentially allowing for dosimetric in-vivo accuracy assessments. Comparison to other techniques and investigations of the short- and long-term clinical impact require further research.
Zusammenfassung
Zielsetzung
Zur Beurteilung gesunder Leberreaktionen nach robotergestützter SBRT untersuchten wir frühzeitige morphologische Veränderungen im MRT (Magnetresonanztomographie) hinsichtlich Patienten- und Behandlungsplanparameter.
Patienten und Methoden
MRT-Daten von 22 Patienten von 42 Lebermetastasen wurden 6–17 Wochen nach Behandlung retrospektiv analysiert. Die mediane Verschreibungsdosis betrug 40 Gy in 3–5 Fraktionen. T2- und T1-gewichtete MRT-Sequenzen wurden zum Bestrahlungsplan registriert. Absolute Dosen wurden in EQD2 mit α/β-Verhältnissen von 2 und 3 Gy für die Modellierung von gesundem und 8 Gy für die von vorgeschädigtem Lebergewebe umgerechnet.
Ergebnisse
Scharf definierte, zumeist zentroidförmige morphologische Veränderungen wurden außerhalb des Hochdosisvolumens um das ursprüngliche GTV herum beobachtet. Hyperintensitäten auf T2-gewichtetem MRT bei EQD2-Isodosen von 113,3 ± 66,1 Gy2, 97,5 ± 54,7 Gy3 und 66,5 ± 32,0 Gy8 hingen signifikant von PTV-Dimension (p = 0,02) und gesundem Leber-EQD2 (p = 0,05) ab. Hypointensitäten auf T1-gewichtetem non-Kontrast-MRT bei EQD2-Isodosen von 113,3 ± 49,3 Gy2, 97,4 ± 41,0 Gy3 und 65,7 ± 24,2 Gy8 waren signifikant von vorheriger Chemotherapie (p = 0,01) und dem Gesamtlebervolumen (p = 0,05) abhängig. Hypointensitäten auf T1-gewichtetem Gadolinium-Kontrast-MRT bei EQD2-Isodosen von 90,6 ± 42,5 Gy2, 79,3 ± 35,3 Gy3 und 56,6 ± 20,9 Gy8 hingen signifikant von Gesamtlebervolumen (p = 0,04) und Volumen der gesunden Leber-EQD2 (p = 0,01) ab.
Schlussfolgerung
Nach robotergestützter SBRT konnten frühe postradiogene Veränderungen des gesunden Lebergewebes räumlich zu den entsprechenden Isodosislinien korreliert werden. Mediane nominale Fraktionsdosen von 10,1–11,3 Gy (EQD2 79–97 Gy3) induzieren charakteristische morphologische Veränderungen um die bestrahlten Läsionen, die möglicherweise dosimetrische In-vivo-Genauigkeitsanalysen erlauben. Der Vergleich zu anderen Behandlungsmethoden und Untersuchungen zu klinischen Kurz- und Langzeitfolgen stehen noch aus.


Similar content being viewed by others
References
Sterzing F, Brunner TB, Ernst I et al (2014) Stereotactic body radiotherapy for liver tumors: principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190(10):872–881
Boda-Heggemann J, Dinter D, Weiss C et al (2012) Hypofractionated image-guided breath-hold SABR (stereotactic ablative body radiotherapy) of liver metastases—clinical results. Radiat Oncol 7:92
Andratschke N, Parys A, Stadtfeld S et al (2016) Clinical results of mean GTV dose optimized robotic guided SBRT for liver metastases. Radiat Oncol 11:74
Klement RJ, Guckenberger M, Alheid H et al (2017) Stereotactic body radiotherapy for oligo-metastatic liver disease – influence of pre-treatment chemotherapy and histology on local tumor control. Radiother Oncol 123(2):227–233
Osmundson EC, Wu Y, Luxton G et al (2015) Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys 91(5):986–994
Toesca DAS, Osmundson EC, von Eyben R et al (2017) Assessment of hepatic function decline after stereotactic body radiation therapy for primary liver cancer. Pract Radiat Oncol 7(3):173–182
Grimm J, LaCouture T, Croce R et al (2011) Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 12(2):3368
LaCouture TA, Xue J, Subedi G et al (2016) Small bowel dose tolerance for stereotactic body radiation therapy. Semin Radiat Oncol 26(2):157–164
Goldsmith C, Price P, Cross T et al (2016) Dose-volume histogram analysis of stereotactic body radiotherapy treatment of pancreatic cancer: a focus on duodenal dose constraints. Semin Radiat Oncol 26(2):149–156
Pan CC, Kavanagh BD, Dawson LA et al (2010) Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 76(3):94–100
Jung J, Yoon SM, Kim SY et al (2013) Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol 8:249
Dreher C, Hoyer KI, Fode MM et al (2016) Metabolic liver function after stereotactic body radiation therapy for hepatocellular carcinoma. Acta Oncol 55(7):886–891
Barry A, McPartlin A, Lindsay P et al (2017) Dosimetric analysis of liver toxicity after liver metastasis stereotactic body radiation therapy. Pract Radiat Oncol 7(5):e331–e337. https://doi.org/10.1016/j.prro.2017.03.004
Herfarth KK, Hof H, Bahner ML et al (2003) Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys 57:444–451
Seidensticker M, Seidensticker R, Mohnike K et al (2011) Quantitative in vivo assessment of radiation injury of the liver using Gd-EOB-DTPA enhanced MRI: tolerance dose of small liver volumes. Radiat Oncol 6:40
Seidensticker M, Burak M, Kalinski T et al (2015) Radiation-induced liver damage: correlation of histopathology with hepatobiliary magnetic resonance imaging, a feasibility study. Cardiovasc Intervent Radiol 38:213–221
Seidensticker M, Seidensticker R, Damm R et al (2014) Prospective randomized trial of enoxaparin, pentoxifylline and ursodeoxycholic acid for prevention of radiation-induced liver toxicity. PLoS ONE 9:e112731
Boda-Heggemann J, Attenberger U, Budjan J et al (2016) MRI morphologic alterations after liver SBRT: direct dose correlation with intermodal matching. Strahlenther Onkol 192(9):641–648
Sanuki-Fujimoto N, Takeda A, Ohashi T et al (2010) CT evaluations of focal liver reactions following stereotactic body radiotherapy for small hepatocellular carcinoma with cirrhosis: relationship between imaging appearance and baseline liver function. Br J Radiol 83:1063–1071
Lo SS, Teh BS, Wang JZ et al (2011) Imaging changes after stereotactic body radiation therapy for lung and liver tumors. Expert Rev Anticancer Ther 11:613–620
Howells CC, Stinauer MA, Diot Q et al (2012) Normal liver tissue density dose response in patients treated with stereotactic body radiation therapy for liver metastases. Int J Radiat Oncol Biol Phys 84:e441–446
Jarraya H, Mirabel X, Taieb S et al (2013) Image-based response assessment of liver metastases following stereotactic body radiotherapy with respiratory tracking. Radiat Oncol 8:24
Richter C, Seco J, Hong TS et al (2014) Radiation-induced changes in hepatocyte-specific Gd-EOB-DTPA enhanced MRI: potential mechanism. Med Hypotheses 83:477–481
Sanuki N, Takeda A, Oku Y et al (2014) Threshold doses for focal liver reaction after stereotactic ablative body radiation therapy for small hepatocellular carcinoma depend on liver function: evaluation on magnetic resonance imaging with Gd-EOB-DTPA. Int J Radiat Oncol Biol Phys 88:306–311
Schmid-Tannwald C, Strobl FF, Theisen D et al (2015) Diffusion-weighted MRI before and after robotic radiosurgery (Cyberknife(R)) in primary and secondary liver malignancies: a pilot study. Technol Cancer Res Treat 14:191–199
Lall C, Bhargava P, Sandrasegaran K et al (2015) Three-dimensional conformal radiation therapy in the liver: MRI findings along a time continuum. J Comput Assist Tomogr 39:356–364
Haddad MM, Merrell KW, Hallemeier CL et al (2016) Stereotactic body radiation therapy of liver tumors: post-treatment appearances and evaluation of treatment response: a pictorial review. Abdom Radiol (NY) 41(10):2061–2077
Doi H, Shiomi H, Masai N et al (2016) Threshold doses and prediction of visually apparent liver dysfunction after stereotactic body radiation therapy in cirrhotic and normal livers using magnetic resonance imaging. J Radiat Res 57(3):294–300
Fukugawa Y, Namimoto T, Toya R et al (2017) Radiation-induced liver injury after 3D-conformal radiotherapy for hepatocellular carcinoma: quantitative assessment using Gd-EOB-DTPA-enhanced MRI. Acta Med Okayama 71(1):25–29
Olsen CC, Welsh J, Kavanagh BD et al (2009) Microscopic and macroscopic tumor and parenchymal effects of liver stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 73:1414–1424
Kilby W, Dooley JR, Kuduvalli G et al (2010) The CyberKnife Robotic Radiosurgery System in 2010. Technol Cancer Res Treat 9(5):433–452
Vautravers-Dewas C, Dewas S, Bonodeau F et al (2011) Image-guided robotic stereotactic body radiation therapy for liver metastases: is there a dose response relationship? Int J Radiat Oncol Biol Phys 81(3):e39–47
Méndez Romero A, Verheij J, Dwarkasing RS et al (2012) Comparison of macroscopic pathology measurements with magnetic resonance imaging and assessment of microscopic pathology extension for colorectal liver metastases. Int J Radiat Oncol Biol Phys 82(1):159–166
Chan M, Grehn M, Cremers F et al (2017) Dosimetric implications of residual tracking errors during robotic SBRT of liver metastases. Int J Radiat Oncol Biol Phys 97(4):839–848
Blanck O, Wang L, Baus W et al (2016) Inverse treatment planning for spinal robotic radiosurgery: an international multi-institutional benchmark trial. J Appl Clin Med Phys 17(3):313–330
Malinowski K, McAvoy TJ, George R et al (2013) Maintaining tumor targeting accuracy in real-time motion compensation systems for respiration-induced tumor motion. Med Phys 40(7):71709
Michaely HJ, Morelli JN, Budjan J et al (2013) CAIPIRINHA-Dixon-TWIST (CDT)-volume-interpolated breath-hold examination (VIBE): a new technique for fast time-resolved dynamic 3‑dimensional imaging of the abdomen with high spatial resolution. Invest Radiol 48:590–597
Morelli JN, Michaely HJ, Meyer MM et al (2013) Comparison of dynamic and liver-specific gadoxetic acid contrast-enhanced MRI versus apparent diffusion coefficients. PLoS ONE 8:e61898
Kanai T, Kadoya N, Ito K et al (2014) Evaluation of accuracy of B‑spline transformation-based deformable image registration with different parameter settings for thoracic images. J Radiat Res 55:1163–1170
Ger RB, Yang J, Ding Y et al (2017) Accuracy of deformable image registration on magnetic resonance images in digital and physical phantoms. Med Phys 44(10):5153–5161. https://doi.org/10.1002/mp.12406
Fukumitsu N, Nitta K, Terunuma T et al (2017) Registration error of the liver CT using deformable image registration of MIM Maestro and Velocity AI. Bmc Med Imaging 17(1):30
Milano MT, Constine LS, Okunieff P (2008) Normal tissue toxicity after small field hypofractionated stereotactic body radiation. Radiat Oncol 3:36
Dawson LA, Ten Haken RK (2005) Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 15:279–283
Son SH, Jang HS, Lee H et al (2013) Determination of the alpha/beta ratio for the normal liver on the basis of radiation-induced hepatic toxicities in patients with hepatocellular carcinoma. Radiat Oncol 8:61
Moustakis C, Blanck O, Ebrahimi Tazehmahalleh F et al (2017) Planning benchmark study for SBRT of early stage NSCLC: results of the DEGRO Working Group Stereotactic Radiotherapy. Strahlenther Onkol 193(10):780–790. https://doi.org/10.1007/s00066-017-1151-8
Dollinger M, Jung EM, Beyer L et al (2014) Irreversible electroporation ablation of malignant hepatic tumors: subacute and follow-up CT appearance of ablation zones. J Vasc Interv Radiol 25(10):1589–1594
Cheng W, Xiao L, Ainiwaer A et al (2015) Molecular responses of radiation-induced liver damage in rats. Mol Med Rep 11(4):2592–2600
Tetreau R, Llacer C, Riou O et al (2017) Evaluation of response after SBRT for liver tumors. Rep Pract Oncol Radiother 22:170–175
Yuan Y, Andronesi OC, Bortfeld TR et al (2013) Feasibility study of in vivo MRI based dosimetric verification of proton end-of-range for liver cancer patients. Radiother Oncol 106:378–382
Pollom EL, Chin AL, Diehn M et al (2017) Normal tissue constraints for abdominal and thoracic stereotactic body radiotherapy. Semin Radiat Oncol 27(3):197–208
Acknowledgements
The Velocity software was provided within the framework of a research agreement between the University Medical Center Mannheim and Varian Medical Systems (USA). We especially thank Yvette Bellingan (Varian, USA) for the excellent assistance with the software. We would also kindly thank Dr. Thorsten Peter (Güstrow, Germany) for his significant support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Boda-Heggemann, A. Jahnke, M.K.H. Chan, L.S. Ghaderi Ardekani, P. Hunold, J.P. Schäfer, S. Huttenlocher, S. Wurster, D. Rades, G. Hildebrandt, F. Lohr, J. Dunst, F. Wenz, and O. Blanck declare that they have no competing interests.
Ethical standards
This retrospective study was approved by the local ethics committees of the medical faculty of the universities of Rostock (A2016-0008) for the clinical part and Kiel (D458/17) for the response assessment part.
Additional information
J. Boda-Heggemann and A. Jahnke contributed equally to this publication
Rights and permissions
About this article
Cite this article
Boda-Heggemann, J., Jahnke, A., Chan, M.K.H. et al. Direct dose correlation of MRI morphologic alterations of healthy liver tissue after robotic liver SBRT. Strahlenther Onkol 194, 414–424 (2018). https://doi.org/10.1007/s00066-018-1271-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1271-9