Abstract
Background
Cetuximab (CET) is a potent inhibitor of the epidermal growth factor receptor and has been shown to have activity in squamous cell carcinoma of the head and neck (SCCHN). We conducted a single-arm phase II trial of a combination therapy comprising cisplatin (CIS), CET and hyperfractionated accelerated radiotherapy (HART).
Patients and methods
Patients with UICC stage III or IVA/B, M0 SCCHN were enrolled and treated with an initial dose of CET (400 mg/m2) and then with a weekly dosage of 250 mg/m2 during HART. HART was started with a prescribed dosage of 2.0 Gy per day for 3 weeks, followed by 1.4 Gy twice daily to a total dose of 70.6 Gy to the gross tumour volume. CIS (40 mg/m2) was administered weekly (days 1, 8, 15, 22, 29 and 36). The primary objective of the phase II study was to determine the 2‑year progression-free survival (PFS).
Results
Between November 2007 and November 2010, a total of 74 patients were enrolled in the study, of whom 65 were evaluable (83% were men). Median age was 56 years (range 37–69 years). An Oropharyngeal primary tumour was diagnosed in 49%, T4a,b in 65% and N2/3 in 96% of the patients. Of these patients, 85% were smokers or ex-smokers. Complete remission (CR) was observed in 23 patients (35%). The most common toxicity grade was ≥3, including mucositis (58%) and dysphagia (52%). The 2‑ and 5‑year overall survival rates were 64 and 41%, the 2‑ and 5‑year PFS rates were 45 and 32%, and the 2‑ and 5‑year locoregional control rates were 47 and 33%, respectively.
Conclusion
The combination of weekly CIS with HART plus CET is a feasible regimen for these unfavourable smoking-induced cancers. However, the parallel US study (RTOG 0522) showed no advantage of the enhanced triple therapy compared to chemoradiotherapy alone.
Zusammenfassung
Hintergrund
Cetuximab (CET) ist ein potenter Inhibitor des epidermalen Wachstumsfaktor-Rezeptors, der schon bei Plattenepithelkarzinomen des Kopf-Hals-Bereichs (SCCHN) Wirkung gezeigt hat. Wir führten eine prospektive, einarmige Phase-II-Studie einer Kombinationstherapie mit Cisplatin (CIS), CET und hyperfraktionierter akzelerierter Strahlentherapie (HART) durch.
Patienten und Methoden
Patienten mit einem M0-SCCHN im UICC-Stadium III oder IVA/B wurden eingeschlossen und mit einer CET-Anfangsdosis von 400 mg/m2 und dann einer wöchentlichen Dosis von 250 mg/m2 während HART behandelt. HART wurde mit 2,0 Gy pro Tag für 3 Wochen begonnen und danach mit 2‑mal täglich 1,4 Gy bis zu einer Gesamtdosis von 70,6 Gy auf das Gesamttumorvolumen fortgesetzt. CIS (40 mg/m2) wurde wöchentlich (Tag 1, 8, 15, 22, 29 und 36) verabreicht. Primäres Ziel der Phase-II-Studie war das progressionsfreie 2‑Jahres-Überleben (PFS).
Ergebnisse
Von den zwischen November 2007 und November 2010 74 in die Studie eingeschlossen Patienten waren 65 auswertbar (83 % Männer). Das Durchschnittsalter betrug 56 Jahre (Spanne 37–69 Jahre). Oropharynxkarzinome wurden bei 49 % diagnostiziert, T4a,b bei 65 % und N2/3 bei 96%. Von diesen Patienten waren 85 % Raucher oder Exraucher. Eine komplette Remission erreichten 23 Patienten (35 %). Die häufigsten Toxizitäten ≥ Grad 3 waren die Mukositis (58 %) und Dysphagie (52 %). Die 2‑ und 5‑Jahres-Überlebensraten lagen bei 64 % und 41 %, die 2‑ und 5‑Jahres-PFS-Raten bei 45 % und 32 % und die lokoregionale Kontrolle nach 2 und 5 Jahren bei 47 % und 33 %.
Schlussfolgerung
Die Kombination von wöchentlichem CIS mit HART plus CET ist eine durchführbare Therapie für diese ungünstigen durch das Rauchen verursachten Karzinome. Die parallel durchgeführte US-Studie (RTOG 0522) ergab aber keinen Vorteil der erweiterten Tripeltherapie im Vergleich zur alleinigen Radiochemotherapie.

Similar content being viewed by others
References
Gupta B, Johnson NW, Kumar N (2016) Global epidemiology of head and neck cancers: a continuing challenge. Oncology 91:13–23
MACH-NC Collaborative Group, Pignon J‑P, le Maître A, Maillard E et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14
Bourhis J, Overgaard J, Audry H et al (2006) Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 368:843–854
Bonner JA, Harari PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Rosenthal DI, Harari PM, Giralt J et al (2016) Association of human Papillomavirus and p16 status with outcomes in the IMCL-9815 phase III registration trial for patients with Locoregionally advanced Oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy with or without Cetuximab. J Clin Oncol 34:1300–1308
Curran D, Giralt J, Harari PM et al (2007) Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol 25:2191–2197
Vermorken JB, Mesia R, Rivera F et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127
Pfister DG, Su YB, Kraus DH et al (2006) Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol 24:1072–1078
Kuhnt T, Sandner A, Wendt T et al (2010) Phase I trial of dose-escalated cisplatin with concomitant cetuximab and hyperfractionated accelerated radiotherapy in locally advanced squamous cell carcinoma of the head and neck. Ann Oncol 21:2284–2289
Sandler AB (2006) Nondermatologic adverse events associated with anti-EGFR therapy. Oncology 20:35–40
Bernier J, Bonner J, Vermorken JB et al (2008) Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol 19:142–149
Dornoff N, Weiß C, Rödel F et al (2015) Re-irradiation with cetuximab or cisplatin-based chemotherapy for recurrent squamous cell carcinoma of the head and neck. Strahlenther Onkol 191:656–664
Kao J, Genden EM, Gupta V et al (2011) Phase 2 trial of concurrent 5‑fluorouracil, hydroxyurea, cetuximab, and hyperfractionated intensity-modulated radiation therapy for locally advanced head and neck cancer. Cancer 117:318–326
Merlano M, Russi E, Benasso M et al (2011) Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: a phase II clinical study. Ann Oncol 22:712–717
Suntharalingam M, Kwok Y, Goloubeva O et al (2012) Phase II study evaluating the addition of cetuximab to the concurrent delivery of weekly carboplatin, paclitaxel, and daily radiotherapy for patients with locally advanced squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 82:1845–1850
Fury MG, Sherman EJ, Rao SS et al (2014) Phase I study of weekly nab-paclitaxel + weekly cetuximab + intensity-modulated radiation therapy (IMRT) in patients with stage III-IVB head and neck squamous cell carcinoma (HNSCC). Ann Oncol 25:689–694
Ang KK, Zhang Q, Rosenthal DI et al (2014) Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 32:2940–2950
Birnbaum A, Dipetrillo T, Rathore R et al (2014) Cetuximab, paclitaxel, carboplatin, and radiation for head and neck cancer: a survival analysis of a Brown University Oncology Group phase II study. Am J Clin Oncol 37:162–166
Egloff AM, Lee JW, Langer CJ et al (2014) Phase II study of Cetuximab in combination with Cisplatin and radiation in unresectable, locally advanced head and neck squamous cell carcinoma: Eastern Cooperative Oncology Group Trial E3303. Clin Cancer Res 20:5041–5051
Nishimura G, Taguchi T, Takahashi M et al (2016) Phase II trial of concurrent bio-chemoradiotherapy using docetaxel, cisplatin, and cetuximab for locally advanced head and neck squamous cell carcinoma. N Cancer Chemother Pharmacol 77:1315–1319
Bourhis J, Sun XS, Sire C et al (2016) Cetuximab-radiotherapy versus cetuximab-radiotherapy plus concurrent chemotherapy in patients with N0-N2a squamous cell carcinoma of the head and neck (SCCHN): Results of the GORTEC 2007-01 phase III randomized trial. J Clin Oncol 34(suppl):abstr 6003
Weinberger PM, Yu Z, Haffty BG et al (2006) Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favourable prognosis. J Clin Oncol 24:736–747
Heiduschka G, Grah A, Oberndorfer F et al (2015) Improved survival in HPV/p16-positive oropharyngeal cancer patients treated with postoperative radiotherapy. Strahlenther Onkol 191:209–216
Acknowledgements
We thank the patients and their families and the medical staff at the various centres who contributed to the patients’ care. We also thank the coordinating centre of the clinical studies at the Martin Luther University Halle-Wittenberg for data monitoring and the Merck KGaA (Darmstadt, Germany) for providing the study medication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Kuhnt, A. Schreiber, A. Pirnasch, M.G. Hautmann, P. Hass, F.P. Sieker, R. Engenhart-Cabillic, M. Richter, K. Dellas and J. Dunst declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Clinical trial number: ISRCTN47339346
Caption Electronic Supplementary Material
66_2017_1145_MOESM1_ESM.docx
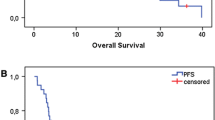
Fig. 2 CONSORT flow chart showing phase I and II. Fig. 3 Median locoregional progression-free survival (LPFS) in months with 95% confidence interval (CI) 18.0 [10.8, 42.1], censored n = 25, intention to treat (ITT) n = 65. Fig. 4 Median overall survival (OS) in months with 95% confidence interval (CI) 40.1 [24.8,not reached]; censored n = 30; intention to treat (ITT) n = 65
Rights and permissions
About this article
Cite this article
Kuhnt, T., Schreiber, A., Pirnasch, A. et al. Hyperfractionated accelerated radiation therapy plus cetuximab plus cisplatin chemotherapy in locally advanced inoperable squamous cell carcinoma of the head and neck. Strahlenther Onkol 193, 733–741 (2017). https://doi.org/10.1007/s00066-017-1145-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1145-6