Abstract
Purpose
To design and apply a framework for predicting symptomatic radiation pneumonitis in patients undergoing thoracic radiation, using both pretreatment anatomic and perfused lung dose–volume parameters.
Materials and methods
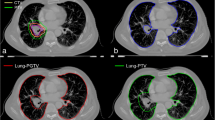
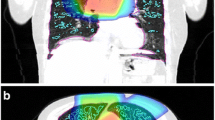
Radiation treatment planning CT scans were coregistered with pretreatment [99mTc]MAA perfusion SPECT/CT scans of 20 patients who underwent definitive thoracic radiation. Clinical radiation pneumonitis was defined as grade ≥ 2 (CTCAE v4 grading system). Anatomic lung dose–volume parameters were collected from the treatment planning scans. Perfusion dose–volume parameters were calculated from pretreatment SPECT/CT scans. Equivalent doses in 2 Gy per fraction were calculated in the lung to account for differences in treatment regimens and spatial variations in lung dose (EQD2lung).
Results
Anatomic lung dosimetric parameters (MLD) and functional lung dosimetric parameters (pMLD70%) were identified as candidate predictors of grade ≥ 2 radiation pneumonitis (AUC > 0.93, p < 0.01). Pairing of an anatomic and functional dosimetric parameter (e. g., MLD and pMLD70%) may further improve prediction accuracy. Not all individuals with high anatomic lung dose (MLD > 13.6 GyEQD2lung, 19.3 Gy for patients receiving 60 Gy in 30 fractions) developed radiation pneumonitis, but all individuals who also had high mean dose to perfused lung (pMLD70% > 13.3 GyEQD2) developed radiation pneumonitis.
Conclusions
The preliminary application of this framework revealed differences between anatomic and perfused lung dosimetry in this limited patient cohort. The addition of perfused lung parameters may help risk stratify patients for radiation pneumonitis, especially in treatment plans with high anatomic mean lung dose. Further investigations are warranted.
Zusammenfassung
Ziel
Erstellung und Anwendung eines Rahmenwerks zur Vorhersage symptomatischer Strahlenpneumonitis bei Patienten mit einer Thorax-Bestrahlung anhand anatomischer und perfundierter Lungendosis-Volumen-Parameter in der Vorbehandlung.
Material und Methoden
CT-Scans zur Bestrahlungsplanung wurden zusammen mit in der Vorbehandlung durchgeführten [99mTc]MAA-SPECT/CT-Perfusionsscans von 20 Patienten mit definitiver Thorax-Bestrahlung aufgezeichnet. Klinische Strahlenpneumonitis wurde als Grad ≥ 2 definiert (CTCAEv4-Gradierung). Anatomische Lungendosis-Volumen-Parameter wurden mittels Behandlungsplanungsscans erhoben und perfundierte Dosis-Volumen-Parameter mittels SPECT/CT-Scans während der Vorbehandlung berechnet. Gleichwertige Dosen von 2 Gy/Fraktion wurden in der Lunge berechnet, um abweichende Behandlungsregime und räumliche Schwankungen der lungengängigen Dosis (EQD2lung) auszugleichen.
Ergebnisse
Anatomische (MLD) und funktionale Parameter der Lungendosis (pMLD70%) wurden als Prädiktoren einer Strahlenpneumonitis vom Grad ≥ 2 (AUC > 0,93; p < 0,01) bestimmt. Gepaarte anatomische und funktionale dosimetrische Parameter (z. B. MLD und pMLD70%) könnten die Vorhersagegenauigkeit weiter erhöhen. Nicht alle Patienten mit hoher anatomischer Lungendosis (MLD > 13,6 GyEQD2lung; 19,3 Gy bei Patienten mit 60 Gy in 30 Fraktionen) entwickelten eine Strahlenpneumonitis, jedoch alle, die zur perfundierten Lunge zudem eine hohe mittlere Dosis aufwiesen (pMLD70% > 13,3 GyEQD2).
Schlussfolgerung
Die vorläufige Anwendung des Rahmenwerks ergab bei dem begrenzten Patientenkollektiv Unterschiede zwischen anatomischer und perfundierter Lungendosimetrie. Zusätzliche Parameter der perfundierten Lunge könnten bei der Risikostratifizierung von Patienten bezüglich einer Strahlenpneumonitis helfen, insbesondere bei Behandlungsplänen mit hoher anatomischer Lungendosis. Weitere Untersuchungen sind nötig.





Similar content being viewed by others
References
Mehta V (2005) Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 63:5–24
Palma DA, Senan S, Tsujino K et al (2013) Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 85:444–450
Marks LB, Bentzen SM, Deasy JO et al (2010) Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 76:S70–76
Graham MV, Purdy JA, Emami B et al (1999) Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 45:323–329
Barriger RB, Fakiris AJ, Hanna N, Yu M, Mantravadi P, McGarry RC (2010) Dose-volume analysis of radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent cisplatinum and etoposide with or without consolidation docetaxel. Int J Radiat Oncol Biol Phys 78:1381–1386
Marks LB, Spencer DP, Sherouse GW et al (1995) The role of three dimensional functional lung imaging in radiation treatment planning: the functional dose-volume histogram. Int J Radiat Oncol Biol Phys 33:65–75
Evans ES, Hahn CA, Kocak Z, Zhou SM, Marks LB (2007) The role of functional imaging in the diagnosis and management of late normal tissue injury. Semin Radiat Oncol 17:72–80
Miften MM, Das SK, Su M, Marks LB (2004) Incorporation of functional imaging data in the evaluation of dose distributions using the generalized concept of equivalent uniform dose. Phys Med Biol 49:1711–1721
Seppenwoolde Y, Engelsman M, De Jaeger K et al (2002) Optimizing radiation treatment plans for lung cancer using lung perfusion information. Radiother Oncol 63:165–177
Lind PA, Marks LB, Hollis D et al (2002) Receiver operating characteristic curves to assess predictors of radiation-induced symptomatic lung injury. Int J Radiat Oncol Biol Phys 54:340–347
Petersson J, Sanchez-Crespo A, Larsson SA, Mure M (1985) Physiological imaging of the lung: single-photon-emission computed tomography (SPECT). J Appl Physiol 2007(102):468–476
Patton JA, Turkington TG (2008) SPECT/CT physical principles and attenuation correction. J Nucl Med Technol 36:1–10
Bailey DL, Willowson KP (2014) Quantitative SPECT/CT: SPECT joins PET as a quantitative imaging modality. Eur J Nucl Med Mol Imaging 41(Suppl 1):S17–25
Bailey DL, Willowson KP (2013) An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med 54:83–89
Mirnezami R, Nicholson J, Darzi A (2012) Preparing for precision medicine. N Engl J Med 366:489–491
Farr KP, Kallehauge JF, Moller DS et al (2015) Inclusion of functional information from perfusion SPECT improves predictive value of dose-volume parameters in lung toxicity outcome after radiotherapy for non-small cell lung cancer: A prospective study. Radiother Oncol 117:9–16
Farr KP, Kramer S, Khalil AA, Morsing A, Grau C (2015) Role of perfusion SPECT in prediction and measurement of pulmonary complications after radiotherapy for lung cancer. Eur J Nucl Med Mol Imaging 42:1315–1324
Borst GR, Ishikawa M, Nijkamp J et al (2010) Radiation pneumonitis after hypofractionated radiotherapy: evaluation of the LQ(L) model and different dose parameters. Int J Radiat Oncol Biol Phys 77:1596–1603
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57:289–300
Marks LB, Sherouse GW, Munley MT, Bentel GC, Spencer DP (1999) Incorporation of functional status into dose-volume analysis. Med Phys 26:196–199
Schytte T, Bentzen SM, Brink C, Hansen O (2015) Changes in pulmonary function after definitive radiotherapy for NSCLC. Radiother Oncol 117:23–28
Hoover DA, Reid RH, Wong E et al (2014) SPECT-based functional lung imaging for the prediction of radiation pneumonitis: a clinical and dosimetric correlation. J Med Imaging Radiat Oncol 58:214–222
Wang D, Sun J, Zhu J, Li X, Zhen Y, Sui S (2012) Functional dosimetric metrics for predicting radiation-induced lung injury in non-small cell lung cancer patients treated with chemoradiotherapy. Radiat Oncol 7:69
Farr KP, Moller DS, Khalil AA, Kramer S, Morsing A, Grau C (2015) Loss of lung function after chemo-radiotherapy for NSCLC measured by perfusion SPECT/CT: Correlation with radiation dose and clinical morbidity. Acta Oncol 54:1350–1354
Robbins ME, Brunso-Bechtold JK, Peiffer AM, Tsien CI, Bailey JE, Marks LB (2012) Imaging radiation-induced normal tissue injury. Radiat Res 177:449–466
Siva S, Devereux T, Ball DL et al (2016) Ga-68 MAA perfusion 4D-PET/CT scanning allows for functional lung avoidance using conformal radiation therapy planning. Technol Cancer Res Treat 15:114–121
McGuire SM, Marks LB, Yin FF, Das SK (2010) A methodology for selecting the beam arrangement to reduce the intensity-modulated radiation therapy (IMRT) dose to the SPECT-defined functioning lung. Phys Med Biol 55:403–416
St-Hilaire J, Lavoie C, Dagnault A et al (2011) Functional avoidance of lung in plan optimization with an aperture-based inverse planning system. Radiother Oncol 100:390–395
Chaudhuri AA, Binkley MS, Rigdon J et al (2016) Pre-treatment non-target lung FDG-PET uptake predicts symptomatic radiation pneumonitis following Stereotactic Ablative Radiotherapy (SABR). Radiother Oncol 119:454–460
Funding
This work was financially supported by NIH/NCI R01CA204301, Radiological Society of North America RSCH1405, and the Fred Hutchinson Cancer Center Support Grant for Protocol-Specific Research Support, P30CA015704. We express our gratitude to the Nuclear Medicine and Radiation Oncology staff for assisting with patient setup during all imaging scans.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.R. Bowen declares grants from NIH/NCI and the Radiological Society of North America during the conduct of the study. J. Zeng declares grants from NIH/NCI. G. Dhami, S.A. Patel, and R. Rengan declare that they have no competing interests. R.S. Miyaoka and H.J. Vesselle declare grants from Philips Healthcare outside of the submitted work. P.E. Kinahan declares a commercial interest as a cofounder of PET/X, LLC, and grants from GE Healthcare outside of the submitted work.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Dhami, G., Zeng, J., Vesselle, H.J. et al. Framework for radiation pneumonitis risk stratification based on anatomic and perfused lung dosimetry. Strahlenther Onkol 193, 410–418 (2017). https://doi.org/10.1007/s00066-017-1114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1114-0