Abstract
Purpose
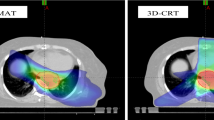
Volumetric-modulated arc therapy (VMAT) achieves high conformity to the planned target volume (PTV) and good sparing of organs at risk (OAR). This study compares dosimetric parameters and toxicity in esophageal cancer (EC) patients treated with VMAT and 3D conformal radiotherapy (3D-CRT).
Materials and methods
Between 2007 and 2014, 17 SC patients received neoadjuvant chemoradiation (CRT) with VMAT. Dose–volume histograms and toxicity were compared between these patients and 20 treated with 3D-CRT. All patients were irradiated with a total dose of 45 Gy. All VMAT patients received simultaneous chemotherapy with cisplatin and 5‑fluorouracil (5-FU) in treatment weeks 1 and 5. Of 20 patients treated with 3D-CRT, 13 (65 %) also received CRT with cisplatin and 5‑FU, whereas 6 patients (30 %) received CRT with weekly oxaliplatin and cetuximab, and a continuous infusion of 5‑FU (OE-7).
Results
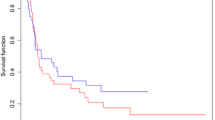
There were no differences in baseline characteristics between the treatment groups. For the lungs, VMAT was associated with a higher V5 (median 90.1 % vs. 79.7 %; p = 0.013) and V10 (68.2 % vs. 56.6 %; p = 0.014), but with a lower V30 (median 6.6 % vs. 11.0 %; p = 0.030). Regarding heart parameters, VMAT was associated with a higher V5 (median 100.0 % vs. 91.0 %; p = 0.043), V10 (92.0 % vs. 79.2 %; p = 0.047), and Dmax (47.5 Gy vs. 46.3 Gy; p = 0.003), but with a lower median dose (18.7 Gy vs. 30.0 Gy; p = 0.026) and V30 (17.7 % vs. 50.4 %; p = 0.015). Complete resection was achieved in 16 VMAT and 19 3D-CRT patients. Due to systemic progression, 2 patients did not undergo surgery. The most frequent postoperative complication was anastomosis insufficiency, occurring in 1 VMAT (6.7 %) and 5 3D-CRT patients (27.8 %; p = 0.180). Postoperative pneumonia was seen in 2 patients of each group (p = 1.000). There was no significant difference in 3‑year overall (65 % VMAT vs. 45 % 3D-CRT; p = 0.493) or 3‑year progression-free survival (53 % VMAT vs. 35 % 3D-CRT; p = 0.453).
Conclusion
Although dosimetric differences in lung and heart exposure were observed, no clinically relevant impact was detected in either patient group. In a real-life patient cohort, VMAT enables reduction of lung and heart V30 compared to 3D-CRT, which may contribute to reduced toxicity.
Zusammenfassung
Ziel
Die volumetrisch modulierte Rotationstherapie (VMAT) erreicht eine hohe Abdeckung des Planungszielvolumens mit guter Schonung der Risikoorgane. Verglichen wurden dosimetrische Parameter und Toxizität zwischen VMAT und 3D-konformaler Strahlentherapie (3D-CRT) bei Patienten mit Ösophaguskarzinom.
Material und Methoden
Zwischen 2007 und 2014 erhielten 17 Patienten während der neoadjuvanten Radiochemotherapie eine VMAT. Dosis-Volumen-Histogramme und Toxizität wurden mit 20 mit 3D-CRT behandelten Patienten verglichen. Die Gesamtdosis aller Patienten betrug 45 Gy (Tagesdosis 1,8 Gy). Die VMAT-Gruppe erhielt eine simultane Chemotherapie mit Cisplatin und 5‑Fluoruracil (5-FU) in der 1. und 5. Woche. In der 3D-CRT-Gruppe bekamen 13 Patienten (65%) ebenfalls eine Chemotherapie mit Cisplatin und 5‑FU, 6 Patienten (30%) eine wöchentliche Chemotherapie mit Oxaliplatin und Cetuximab, sowie eine kontinuierliche Chemotherapie mit 5‑FU (OE-7).
Ergebnisse
Hinsichtlich der Basischarakteristika zeigte sich kein Unterschied zwischen den Gruppen. Für die Lungen war VMAT mit einer höheren V5 (Median 90,1% vs. 79,7%; p = 0,013) und V10 (68,2% vs. 56,6%; p = 0,014), aber einer niedrigeren V30 assoziiert (Median 6,6% vs. 11,0%; p = 0,030). Für das Herz waren für VMAT V5 (Median 100 % vs. 91 %; p = 0,043), V10 (92 % vs. 79,2 %, p = 0,047) und Dmax (47,5 Gy vs. 46,3 Gy; p = 0,003) höher, die mittlere Dosis (18,7 Gy vs. 30 Gy, p = 0,026) und V30 (17,7 % vs. 50,4 %; p = 0,015) niedriger. Bei 16 VMAT- bzw. 19 3D-CRT-Patienten wurde der Tumor komplett reseziert. Aufgrund eines systemischen Progresses wurden 2 Patienten nicht operiert. Häufigste postoperative Komplikation war die Anastomoseninsuffizienz bei einem VMAT- (6,7 %) und 5 3D-CRT-Patienten (27,8 %; p = 0,180). In jeder Gruppe entwickelten 2 Patienten eine postoperative Pneumonie (p = 1,000). Beim 3‑Jahres-Gesamt- (65 % vs. 45 %; p = 0,493) und progressionsfreien Überleben (53 % vs. 35 %; p = 0,453) zeigte sich kein Unterschied. Ein Lokalrezidiv wurde bei 6 3D-CRT- (30 %) und bei einem VMAT-Patienten (6 %) beobachtet (p = 0,097).
Schlussfolgerung
Dosimetrische Unterschiede der Dosisverteilung in Herz und Lungen waren klinisch nicht relevant. Verglichen mit 3D-CRT reduziert VMAT V30 für Lunge und Herz und somit möglicherweise die Toxizität.


Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24(14):2137–2150
Zhang Y (2013) Epidemiology of esophageal cancer. World J Gastroenterol 19(34):5598–5606
Kranzfelder M et al (2011) Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg 98(6):768–783
Shapiro J et al (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol 16(9):1090–1098
Sjoquist KM et al (2011) Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol 12(7):681–692
Fogliata A et al (2015) A broad scope knowledge based model for optimization of VMAT in esophageal cancer: validation and assessment of plan quality among different treatment centers. Radiat Oncol 10:220
Gong G et al (2013) Reduced lung dose during radiotherapy for thoracic esophageal carcinoma: VMAT combined with active breathing control for moderate DIBH. Radiat Oncol 8:291
Kataria T et al (2014) Dosimetric comparison between Volumetric Modulated Arc Therapy (VMAT) vs Intensity Modulated Radiation Therapy (IMRT) for radiotherapy of mid esophageal carcinoma. J Cancer Res Ther 10(4):871–877
Zhang WZ et al (2015) Volumetric modulated arc therapy vs. c‑IMRT for the treatment of upper thoracic esophageal cancer. PLoS ONE 10(3):e0121385
Nomura M et al (2012) Predictive factors for radiation pneumonitis in oesophageal cancer patients treated with chemoradiotherapy without prophylactic nodal irradiation. Br J Radiol 85(1014):813–818
Kumar G et al (2012) Analysis of dose-volume parameters predicting radiation pneumonitis in patients with esophageal cancer treated with 3D-conformal radiation therapy or IMRT. Jpn J Radiol 30(1):18–24
Hayashi K et al (2015) Predictive factors for pericardial effusion identified by heart dose-volume histogram analysis in oesophageal cancer patients treated with chemoradiotherapy. Br J Radiol 88(1046):20140168
Wei X et al (2008) Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys 70(3):707–714
Lund M et al (2015) Effects on heart function of neoadjuvant chemotherapy and chemoradiotherapy in patients with cancer in the esophagus or gastroesophageal junction – a prospective cohort pilot study within a randomized clinical trial. Radiat Oncol 10(1):16
Umezawa R et al (2015) Assessment of myocardial metabolic disorder associated with mediastinal radiotherapy for esophageal cancer –a pilot study. Radiat Oncol 10:96
Kuo AH et al (2015) Cardiac and inflammatory biomarkers do not correlate with volume of heart or lung receiving radiation. Radiat Oncol 10(1):5
Cozzi L et al (2008) A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiother Oncol 89(2):180–191
Wolff D et al (2009) Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol 93(2):226–233
Zhang T et al (2015) Double-arc volumetric modulated therapy improves dose distribution compared to static gantry IMRT and 3D conformal radiotherapy for adjuvant therapy of gastric cancer. Radiat Oncol 10:114
Wang X et al (2013) Single-arc volumetric-modulated arc therapy (sVMAT) as adjuvant treatment for gastric cancer: dosimetric comparisons with three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT). Med Dosim 38(4):395–400
Liu GF et al (2014) Clinical outcomes for gastric cancer following adjuvant chemoradiation utilizing intensity modulated versus three-dimensional conformal radiotherapy. PLoS ONE 9(1):e82642
Fakhrian K et al (2013) Advanced techniques in neoadjuvant radiotherapy allow dose escalation without increased dose to the organs at risk : Planning study in esophageal carcinoma. Strahlenther Onkol 189(4):293–300
Zhao Y et al (2015) Predictive factors for acute radiation pneumonitis in postoperative intensity modulated radiation therapy and volumetric modulated arc therapy of esophageal cancer. Thorac Cancer 6(1):49–57
Lin CY et al (2014) Dosimetric and efficiency comparison of high-dose radiotherapy for esophageal cancer: volumetric modulated arc therapy versus fixed-field intensity-modulated radiotherapy. Dis Esophagus 27(6):585–590
Feng M et al (2011) Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 79(1):10–18
Fiandra C et al (2012) Different IMRT solutions vs. 3D-conformal radiotherapy in early stage Hodgkin’s Lymphoma: Dosimetric comparison and clinical considerations. Radiat Oncol 7:186
Minn AY et al (2010) Comparison of intensity-modulated radiotherapy and 3‑dimensional conformal radiotherapy as adjuvant therapy for gastric cancer. Cancer 116(16):3943–3952
Wang SL et al (2006) Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 64(3):692–699
Martel MK et al (1998) Fraction size and dose parameters related to the incidence of pericardial effusions. Int J Radiat Oncol Biol Phys 40(1):155–161
Gagliardi G et al (2010) Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 76(3 Suppl):S77–S85
Chandra A et al (2005) Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol 77(3):247–253
Boda-Heggemann J et al (2013) Adjuvant IMRT/XELOX radiochemotherapy improves long-term overall- and disease-free survival in advanced gastric cancer. Strahlenther Onkol 189(5):417–423
Ordu AD et al (2015) Radio(chemo)therapy for locally advanced squamous cell carcinoma of the esophagus: Long-term outcome. Strahlenther Onkol 191(2):153–160
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Münch, S. Aichmeier, A. Hapfelmeier, M.-N. Duma, M. Oechsner, M. Feith, S.E. Combs, and D. Habermehl declare that they have no competing interests.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The local ethics committee supported and approved the retrospective analysis of the included patients (project number: 288/15).
Rights and permissions
About this article
Cite this article
Münch, S., Aichmeier, S., Hapfelmeier, A. et al. Comparison of dosimetric parameters and toxicity in esophageal cancer patients undergoing 3D conformal radiotherapy or VMAT. Strahlenther Onkol 192, 722–729 (2016). https://doi.org/10.1007/s00066-016-1020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-016-1020-x