Abstract
Background
The epidermal growth factor receptors, EGFR (HER1) and HER2, have proven prognostic relevance in a variety of human malignancies and both are functionally involved in the molecular pathogenesis of malignant gliomas.
Material and methods
We selectively inhibited EGFR and HER2 in glioblastoma cell lines via EGFR- and HER2-specific siRNAs and through the binding of the therapeutic antibodies cetuximab and trastuzumab. The expression of EGFR and HER2 was verified by real-time PCR and western blot analyses. We examined the growth rate, cell cycle distribution, cell migration, clonogenic survival, and radiosensitivity of U251MG and LN-229 glioblastoma cell lines to determine the physiological and cell biological effects of EGFR and HER2 targeting.
Results
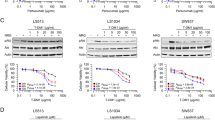
EGFR and HER2 targeting using the therapeutic antibodies cetuximab and trastuzumab had no effect on cellular growth rate, cell cycle distribution, cell migration, clonogenic survival, and radiosensitivity in the cell lines U251 and LN-229. In contrast, siRNA knock-down of EGFR and HER2, reduced the growth rate by 40–65 %. The knock-down of EGFR did not change the cell migration rate in the cell lines U251 and LN-229. However, knock-down of HER2 reduced the cell migration rate by 50 %. Radiobiological analysis revealed that EGFR knock-down induced no radiosensitization in U251MG and LN-229 cells. However, the knock-down of HER2 induced radiosensitization in U251MG cells.
Conclusion
The epidermal growth factor receptor HER2 is a promising anti-tumor target for the therapy of glioblastoma. HER2 targeting may represent a promising strategy to induce cell physiological and radiobiological anti-tumor effects in glioblastoma.
Zusammenfassung
Hintergrund
Die epidermalen Wachstumsfaktorrezeptoren EGFR (HER1) und HER2 haben in verschiedenen humanen Malignitäten eine prognostische Bedeutung und sind funktionell in die molekulare Pathogenese von malignen Gliomen involviert.
Material und Methoden
Wir inhibierten EGFR und HER2 in Glioblastomzelllinien selektiv durch EGFR- und HER2-spezifische siRNAs bzw. durch die Inkubation mit den therapeutischen Antikörpern Cetuximab und Trastuzumab. Die Expression von EGFR und HER2 wurde mittels Real-Time-PCR und Western-Blot-Analysen verifiziert. Um die physiologischen und zellbiologischen Effekte der EGFR- und HER2-Inhibierung zu untersuchen, wurden Zellwachstum, Verteilung der Zellen auf die Zellzyklusphasen, Zellmigration, klonogenes Zellüberleben und Radiosensitivität bestimmt.
Ergebnisse
Die EGFR- und HER2-Inhibierung mit Hilfe der therapeutischen Antikörper Cetuximab und Trastuzumab hatte keinen Effekt auf das Wachstum, die Verteilung der Zellen auf die Zellzyklusphasen, die Zellmigration, das klonogene Zellüberleben und die Radiosensitivität. Im Gegensatz dazu reduzierte der „Knock-down“ von EGFR und HER2 durch die siRNA die Wachstumsrate um 40–65 %. Der „Knock-down“ von EGFR hatte keinen Einfluss auf die Zellmigration der Zelllinien U251MG und LN-229. Der „Knock-down“ von HER2 dagegen reduzierte die Zellmigration um 50 %. In radiobiologischen Untersuchungen zeigte sich keine Radiosensibilisierung in den Zelllinien U251MG und LN-229 nach „Knock-down“ von EGFR. Im Gegensatz dazu induzierte der „Knock-down“ von HER2 eine Radiosensibilisierung in U251MG-Zellen.
Schlussfolgerung
Der epidermale Wachstumsfaktorrezeptor HER2 ist ein vielversprechendes Ziel für eine antitumorale targetorientierte Therapie von Glioblastomen. Die Inhibierung von HER2 scheint dabei eine vielversprechende Strategie zu sein, um zellphysiologische und radiobiologische antitumorale Effekte in Glioblastomen zu induzieren.





Similar content being viewed by others
References
Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109:93–108
van den Bent MJ (2006) Adjuvant treatment of high grade gliomas. Ann Oncol 17:X186–X190
Johns TG, Mckay MJ, Cvrljevic AN et al (2010) Mab 806 Enhances the efficacy of Ionizing radiation in glioma xenografts expressing the De2–7 epidermal growth factor receptor. Int J Radiat Oncol Biol Phys 78:572–578
Fedrigo CA, Grivicich I, Schunemann DP et al (2011) Radioresistance of human glioma spheroids and expression of HSP70, p53 and EGFr. Radiat Oncol 6:156
Mukherjee B, McEllin B, Camacho CV et al (2009) EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res 69:4252–4259
Citri A, Yarden Y (2006) EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7:505–516
Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170:1445–1453
Chakravarti A, Dicker A, Mehta M (2004) The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: a review of preclinical and correlative clinical data. Int J Radiat Oncol Biol Phys 58:927–931
Yarden Y (2001) The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur J Cancer 37:S3–S8
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137
Mineo JF, Bordron A, Quintion-Roue I et al (2006) Increasing of HER2 membranar density in human glioblastoma U251MG cell line established in a new nude mice model. J Neurooncol 76:249–255
Mineo JF, Bordron A, Baroncini M et al (2007) Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J Neurooncol 85:281–287
Moasser MM (2007) The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26:6469–6487
Nicholson RI, Gee JMW, Harper ME (2001) EGFR and cancer prognosis. Eur J Cancer 37:S9–S15
Rego RL, Foster NR, Smyrk TC et al (2010) Prognostic effect of activated EGFR expression in human colon carcinomas: comparison with EGFR status. Br J Cancer 102:165–172
Wichmann H, Guttler A, Bache M et al (2014) Inverse prognostic impact of ErbB2 mRNA and protein expression level in tumors of soft tissue sarcoma patients. Strahlenther Onkol
Slamon DJ, Clark GM, Wong SG et al (1987) Human-breast cancer—correlation of relapse and survival with amplification of the Her-2 Neu oncogene. Science 235:177–182
Andrulis IL, Bull SB, Blackstein ME et al (1998) Neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. J Clin Oncol 16:1340–1349
Shen KR, Zhang SD, Zhao L et al (2014) Role of EGFR as a prognostic factor for survival in head and neck cancer: a meta-analysis. Tumor Biol 35:2285–2295
Sartore-Bianchi A, Moroni M, Veronese S et al (2007) Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol 25:3238–3245
Bergonie J, Tribondeau L (1959) Interpretation of some results of radiotherapy and an attempt at determining a logical technique of treatment [De Quelques Resultats de la Radiotherapie et Essai de Fixation d’une Technique Rationnelle]. Radiat Res 11:587–588
Heinemann V, Stintzing S, Kirchner T et al (2009) Clinical relevance of EGFR- and KRAS-status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat Rev 35:262–271
Mohan S, Heitzer E, Ulz P et al (2014) Changes in colorectal carcinoma genomes under anti-EGFR therapy identified by whole-genome plasma DNA sequencing. PLoS Genet 10:e1004271
Blick SKA, Scott LJ (2007) Cetuximab—a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs 67:2585–2607
Kulkarni S, Hicks DG (2008) HER2-Positive early breast cancer and trastuzumab: A surgeon’s perspective. Ann Surg Oncol 15:1677–1688
Hahnel A, Wichmann H, Kappler M et al (2010) Effects of osteopontin inhibition on radiosensitivity of MDA-MB-231 breast cancer cells. Radiat Oncol 5:82
Bonner JA, Harari PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. New England J Med 354:567–578
Slamon D (2000) Herceptin: increasing survival in metastatic breast cancer. Eur J Oncol Nurs 4:24–29
Hudis CA (2007) Drug therapy: Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med 357:39–51
Schulte A, Liffers K, Kathagen A et al (2013) Erlotinib resistance in EGFR-amplified glioblastoma cells is associated with upregulation of EGFRvIII and PI3Kp110δ. Neuro-Oncol 15:1289–1301
Upez-Albaitero A, Ferris RL (2007) Immune activation by epidermal growth factor receptor-specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg 133:1277–1281
Chen G, Kronenberger P, Teugels E et al (2012) Targeting the epidermal growth factor receptor in non-small cell lung cancer cells: the effect of combining RNA interference with tyrosine kinase inhibitors or cetuximab. Bmc Med 10:28
Combs SE, Schulz-Ertner D, Roth W et al (2007) In vitro responsiveness of glioma cell lines to multimodality treatment with radiotherapy, temozolomide, and epidermal growth factor receptor inhibition with cetuximab. Int J Radiat Oncol Biol Phys 68:873–882
Miqueli AD, Rolff J, Lemm M et al (2009) Radiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodies. Br J Cancer 100:950–958
Dittmann K, Mayer C, Fehrenbacher B et al (2005) Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem 280:31182–31189
Baumann M, Zips D, Krause M (2012) [Experimental tumor therapy]. Strahlenther Onkol 188:291–294
Le XF, Almeida MI, Mao WQ et al (2012) Modulation of MicroRNA-194 and cell migration by HER2-Targeting trastuzumab in breast cancer. Plos One 7:e41170
Yakes FM, Chinratanalab W, Ritter CA et al (2002) Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 62:4132–4141
Longva KE, Pedersen NM, Haslekas C et al (2005) Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int J Cancer 116:359–367
Barok M, Isola J, Palyi-Krekk Z et al (2007) Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther 6:2065–2072
Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S et al (2011) IL-12 enhances the antitumor actions of trastuzumab via NK Cell IFN-gamma production. J Immunol 186:3401–3409
Roda JM, Joshi T, Butchar JP et al (2007) The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor-positive tumor cells is enhanced by cytokines. Clin Cancer Res 13:6419–6428
Garrett JT, Olivares MG, Rinehart C et al (2011) Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A 108:5021–5026
Wolf-Yadlin A, Kumar N, Zhang Y et al (2006) Effects of HER2 overexpression on cell signaling networks governing proliferation and migration. Mol Syst Biol 2:54
Faltus T, Yuan JP, Zimmer B et al (2004) Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia 6:786–795
Choudhury A, Charo J, Parapuram SK et al (2004) Small interfering RNA (siRNA) inhibits the expression of the Her2/neu gene, upregulates HLA class I and induces apoptosis of Her2/neu positive tumor cell lines. Int J Cancer 108:71–77
Vollmann A, Vornlocher HP, Stempfl T et al (2006) Effective silencing of EGFR with RNAi demonstrates non-EGFR dependent proliferation of glioma cells. Int J Oncol 28:1531–1542
Lo HW, Hung MC (2006) Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer 94:184–188
Kang CS, Zhang ZY, Jia ZF et al (2006) Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther 13:530–538
Neve RM, Sutterluty H, Pullen N et al (2000) Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cells. Oncogene 19:1647–1656
Kumar N, Zaman MH, Kim HD et al (2006) A high-throughput migration assay reveals HER2-mediated cell migration arising from increased directional persistence. Biophys J 91:L32–L34
Toulany M, Rodemann HP (2010) Membrane receptor signaling and control of DNA repair after exposure to ionizing radiation. Nuklearmedizin 49:S26–S30
Lammering G, Valerie K, Lin PS et al (2001) Radiosensitization of malignant glioma cells through overexpression of dominant-negative epidermal growth factor receptor. Clin Cancer Res 7:682–690
Gao L, Li FS, Dong B et al (2010) Inhibition of Stat3 and Erbb2 suppresses tumor growth, enhances radiosensitivity, and Induces mitochondria-dependent apoptosis in glioma cells. Int J Radiat Oncol Biol Phys 77:1223–1231
Hasselbalch B, Lassen U, Poulsen HS et al (2010) Cetuximab insufficiently inhibits glioma cell growth due to persistent EGFR downstream signaling. Cancer Invest 28:775–787
Yang CF, Liu Y, Lemmon MA et al (2006) Essential role for Rac in heregulin beta 1 mitogenic signaling: a mechanism that involves epidermal growth factor receptor and is independent of ErbB4. Mol Cell Biol 26:831–842
Ulu N, Henning RH, Guner S et al (2013) Intracellular transactivation of epidermal growth factor receptor by alpha(1A)-Adrenoceptor is mediated by phosphatidylinositol 3-Kinase Independently of activation of extracellular signal regulated kinases 1/2 and Serine-Threonine kinases in Chinese hamster ovary cells. J Pharmacol Exp Ther 347:47–56
Brodowicz T, Wiltschke C, Budinsky AC et al (1997) Soluble HER-2/neu neutralizes biologic effects of anti-HER-2/neu antibody on breast cancer cells in vitro. Int J Cancer 73:875–879
Compliance with ethical guidelines
Conflict of interests
H. Wichmann, A. Güttler, M. Bache, H. Taubert, S. Rot, J. Kessler, A.W. Eckert, M. Kappler, and D. Vordermark state that there are no conflicts of interest. The accompanying manuscript does not include studies on humans or animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Matthias Kappler and Dirk Vordermark contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wichmann, H., Güttler, A., Bache, M. et al. Targeting of EGFR and HER2 with therapeutic antibodies and siRNA. Strahlenther Onkol 191, 180–191 (2015). https://doi.org/10.1007/s00066-014-0743-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0743-9