Abstract
Introduction
The aim of this clinical registry is to record the use of CytoSorb® adsorber device in critically ill patients under real-life conditions.
Methods
The registry records all relevant information in the course of product use, e. g., diagnosis, comorbidities, course of the condition, treatment, concomitant medication, clinical laboratory parameters, and outcome (ClinicalTrials.gov Identifier: NCT02312024). Primary endpoint is in-hospital mortality as compared to the mortality predicted by the APACHE II and SAPS II score, respectively.
Results
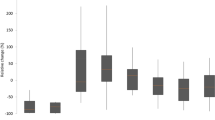
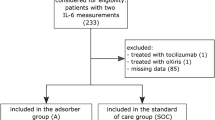
As of January 30, 2017, 130 centers from 22 countries were participating. Data available from the start of the registry on May 18, 2015 to November 24, 2016 (122 centers; 22 countries) were analyzed, of whom 20 centers from four countries provided data for a total of 198 patients (mean age 60.3 ± 15.1 years, 135 men [68.2%]). In all, 192 (97.0%) had 1 to 5 Cytosorb® adsorber applications. Sepsis was the most common indication for CytoSorb® treatment (135 patients). Mean APACHE II score in this group was 33.1 ± 8.4 [range 15–52] with a predicted risk of death of 78%, whereas the observed mortality was 65%. There were no significant decreases in the SOFA scores after treatment (17.2 ± 4.8 [3–24]). However interleukin-6 levels were markedly reduced after treatment (median 5000 pg/ml before and 289 pg/ml after treatment, respectively).
Conclusions
This third interim report demonstrates the feasibility of the registry with excellent data quality and completeness from 20 study centers. The results must be interpreted with caution, since the numbers are still small; however the disease severity is remarkably high and suggests that adsorber treatment might be used as an ultimate treatment in life-threatening situations. There were no device-associated side effects.
Zusammenfassung
Einleitung
Zweck des vorgestellten klinischen Registers ist es, die Nutzung des Adsorbers CytoSorb® bei kritisch kranken Patienten unter Realbedingungen zu erfassen.
Methoden
Dokumentiert werden Diagnose, Komorbiditäten, Verlauf der Erkrankung, Behandlung, Begleitmedikation und Laborparameter (ClinicalTrials.gov-Identifier: NCT02312024). Primärer Endpunkt ist die Krankenhausmortalität im Vergleich zur Mortalität, die mit dem APACHE-II- bzw. SAPS-II-Score prognostiziert wurde.
Ergebnisse
Mit Stand vom 30. Januar 2017 nahmen 130 Zentren aus 22 Ländern am Register teil. Verfügbare Daten vom 18. Mai 2015 bis 24. November 2016 (122 Zentren; 22 Länder) wurden analysiert. Zwanzig dieser Zentren aus 4 Ländern lieferten Daten zu insgesamt 198 Patienten (Alter 60,3 ± 15,1 Jahre, 135 Männer [68,2 %]). Insgesamt 192 (97,0 %) hatten 1–5 Cytosorb®-Adsorber-Anwendungen. Sepsis war die häufigste Indikation zur CytoSorb®-Behandlung (135 Patienten). Der durchschnittliche APACHE-II-Score in dieser Gruppe betrug 33,1 ± 8,4 mit einem prognostizierten Sterberisiko von 78 %, während die beobachtete Mortalität bei 65 % lag. Es fanden sich keine signifikanten Verringerungen in den SOFA-Scores nach Behandlung (17,2 ± 4,8 [3–24]). Die Interleukin-6-Spiegel waren allerdings nach Behandlung deutlich reduziert (im Median 5000 pg/ml vor und 289 pg/ml nach Behandlung).
Schlussfolgerungen
Dieser dritte Zwischenbericht belegt die Machbarkeit des Registers mit einer exzellenten Datenqualität und -vollständigkeit aus 20 Studienzentren. Die Ergebnisse sind mit Zurückhaltung zu interpretieren, da die Patientenzahl immer noch gering ist; die Erkrankungsschwere ist allerdings bemerkenswert hoch, was vermuten lässt, dass der Adsorber als Ultima Ratio genutzt wird. Es gab keine device-assoziierten Nebenwirkungen.




Similar content being viewed by others
References
Suntharalingam G et al (2006) Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355(10):1018–1028
Hotchkiss RS et al (2009) The sepsis seesaw: tilting toward immunosuppression. Nat Med 15(5):496–497
Honore PM, Matson JR (2004) Extracorporeal removal for sepsis: Acting at the tissue level – the beginning of a new era for this treatment modality in septic shock. Crit Care Med 32(3):896–897
Di Carlo JV, Alexander SR (2005) Hemofiltration for cytokine-driven illnesses: the mediator delivery hypothesis. Int J Artif Organs 28(8):777–786
Peng Z et al (2010) Blood purification in sepsis: a new paradigm. Contrib Nephrol 165:322–328
Rimmele T, Kellum JA (2011) Clinical review: blood purification for sepsis. Crit Care 15(1):205
Esteban E et al (2013) Immunomodulation in sepsis: the role of endotoxin removal by polymyxin B‑immobilized cartridge. Mediators Inflamm 2013:507539
Bernardi MH, Rinoesl H, Dragosits K (2016) Effect of hemoadsorption during cardiopulmonary bypass surgery – a blinded, randomized, controlled pilot study using a novel adsorbent. Crit Care 20:96. https://doi.org/10.1186/s13054-016-1270-0
Muller D et al (2010) Memorandum Register für die Versorgungsforschung [Memorandum registry for health services research]. Gesundheitswesen 72(11):824–839
Wegscheider K (2004) Medizinische Register – Nutzen und Grenzen [Medical registries. Benefits and limitations]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 47(5):416–421
Gliklich RE, Dreyer NA (eds) (2010) Registries for evaluating patient outcomes: a user’s guide, 2nd edn. Agency for Healthcare Research and Quality, Rockville MD
Knaus WA et al (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Le Gall RJ et al (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Nashef SAM et al (2012) EuroSCORE II. Eur J Cardiothorac Surg 41:1–12
Funding
Supported with unrestricted grants by CytoSorbents Europe GmbH
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F. Bach, R. Bogdanski, F. Born, E. Grigoryev, H. Haake, D. Jacob, J.T. Kielstein, A. Meier-Hellmann, F. Nestler, M. Nitsch, D. Olboeter, M. Schott, M. Singer and D. Tomescu declare that they have no competing interests. A. Baumann reports grants, personal fees and nonfinancial support from Cytosorbents GmbH, personal fees and nonfinancial support from Diamed GmbH, nonfinancial support and other from Orion Pharma, nonfinancial support and other from MSD Pharma, outside the submitted work. F.M. Brunkhorst reports grants and personal fees from CytoSorbents Europe. S. Friesecke reports grants, personal fees and nonfinancial support from cytosorbents Europe. J. Kellum reports grants and personal fees from Cytostorbenets, outside the submitted work. K. Kogelmann reports grants from Cytostorbenets, outside the submitted work. Z. Molnar reports personal fees from CytoSorbents Europe. A. Nierhaus reports personal fees from CytoSorbents Europe, Personal fees from Biotest AG, outside the submitted work. M. Quintel reports personal fees from Cytosorbent, outside the submitted work. G.A. Schittek reports personal fees from Cytosorbents Europe GmbH, outside the submitted work. U. Schumacher reports personal fees from BAYER, outside the submitted work (part time employee). K. Träger reports personal fees and other from Cytosorbents Europe. A. Weyland reports personal fees and other from CytoSorbents Europe GmbH, outside the submitted work.
This study protocol has been submitted to the Institutional Review Board of the Faculty of Medicine at Friedrich Schiller University, Jena that acts as the IRB in charge for Germany. All German ethic committees involved are informed about the participation of centers in their area of responsibility and the decision of the IRB in charge. In centers from outside Germany, approval of the local ethics commission in charge is obtained and all national regulations are adhered to.
Additional information
Redaktion
M. Buerke, Siegen
Caption Electronic Supplementary Material
63_2017_342_MOESM1_ESM.pdf
1. Flowcharts and data content in the eCRF, 2. Severity scores, 3. Criteria defining sepsis and septic shock, 4. Abbreviations
Rights and permissions
About this article
Cite this article
Friesecke, S., Träger, K., Schittek, G.A. et al. International registry on the use of the CytoSorb® adsorber in ICU patients. Med Klin Intensivmed Notfmed 114, 699–707 (2019). https://doi.org/10.1007/s00063-017-0342-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00063-017-0342-5