Abstract
Purpose
To quantify involvement of globus pallidus and two midbrain nuclei (substantia nigra and red nucleus) in Pantothenate Kinase-Associated Neurodegeneration (PKAN).
Material and Methods
We performed T2 and T2* weighted imaging with calculation of the corresponding relaxation times on a subset of 5 patients from a larger group of 20 patients with PKAN from the southwest part of the Dominican Republic. Examinations were carried out on a 3T scanner and included a multi-echo spin-echo as well as a multi-echo gradient echo sequence. Results were compared to a control group of 19 volunteers.
Results
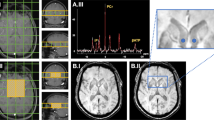
T2 and T2* weighted sequences showed abnormal signal reduction in the globus pallidus of all patients. On T2* weighted imaging, abnormal signal in the substantia nigra could reliably be detected in 75% of cases, but differentiation from normal was less reliable in T2 weighted scans. Correspondingly, relaxation times differed from normal with very high significance (p < 0.0001) in the globus pallidus, but with with less significance in the substantia nigra (p ≤ 0.03). The red nucleus was not affected.
Conclusions
Signal reduction in the globus pallidus, which probably is due to abnormal accumulation of iron, is severe in PKAN and can be differentiated from normal with high reliability. The substantia nigra is affected to a lesser degree, and the red nucleus is not involved. The reason for this selective susceptibility of normally iron-rich brain structures for pathological accumulation of iron remains speculative. Our quantitative results might be helpful to assess the value of an iron chelation approach to therapy.

Similar content being viewed by others
References
Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, et al. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med. 2003;348:33–40.
Pellecchia MT, Valente EM, Cif L, Salvi S, Albanese A, Scarano V, et al. The diverse phenotype and genotype of pantothenate kinase-associated neurodegeneration. Neurology. 2005;64:1810–12.
Delgado RF, Sanchez PR, Speckter H, Then EP, Jimenez R, Oviedo J, Dellani PR, Foerster B, Stoeter P. Missense PANK2 mutation without “eye of the tiger sign”—MR findings in a large group of patients with Pantothenate Kinase-Associated Neurodegeneration (PKAN). J Magn Reson Imaging. 2011 [Epub ahead of print].
Senegas J, Bos C, Dahnke H. Determining precision of relaxation time measurements: application to T2 mapping. ISMRM 2008; 16th Scientific Meeting, Toronto/Can. Proceedings 2008, p. 1421.
Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage. 2009;47:493–500.
Peran P, Cherubini A, Luccichenti G, Hagberg G, Démonet JF, Rascol O, et al. Volume and iron content in basal ganglia and thalamus. Hum Brain Mapp. 2009;30:2667–75.
Langkammer C, Enzinger C, Quasthoff S, Grafenauer P, Soellinger M, Fazekas F, et al. Mapping of iron deposition in conjunction with assessment of nerve fiber tract integrity in amyotrophic lateral sclerosis. J Magn Reson Imaging. 2010;31:1339–45.
Szumowski J, Bas E, Gaarder K, Schwarz E, Erdogmus D, Hayflick S. Measurement of brain iron distribution in Hallervorden-Spatz syndrome. J Magn Reson Imaging. 2010;31:482–9.
Helms G, Draganski B, Frackowiak R, Ashburner J, Weiskopf N. Improved segmentation of deep brain grey matter structures using magnetization transfer parameter maps. Neuroimage. 2009;47:194–8.
Vyzmal J, Hajek M, Patronas N, Gledd JN, Bulte JW, Baumgarner C, et al. The quantitative relation between T1-weighted and T2-weighted MRI of normal grey matter and iron concentration. J Magn Reson Imaging. 1995;5:554–60.
Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23:1–25.
Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, et al. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252:165–72.
Sullivan EV, Adalsteinsson E, Rohlfing T, Pfefferbaum A. Relevance of iron deposition in deep gray matter brain structures to cognitive and motor performance in healthy elderly men and women: exploratory findings. Brain Imaging Behav. 2009;3:167–75.
Bartzokis G, Lu PH, Tishler TA, Peters DG, Kosenko A, Barrall KA, et al. Prevalent iron metabolism gene variants associated with increased brain ferritin iron in healthy older men. J Alzheimers Dis. 2010;20:333–41.
Ge Y, Jensen JH, Lu H, Helpern JA, Miles L, Inglese M, et al. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. AJNR Am J Neuroradiol. 2007;28:1639–44.
Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–86.
Hayflick SJ, Hartman M, Coryell J, Gitschier J, Rowley H. Brain MRI in neurodegeneration with brain iron accumulation with and without PANK2 mutations. AJNR Am J Neuroradiol. 2006;27:1230–3.
Gupta R, Kumar A, Sharma MC, Sarkar C, Goyal V, Bihari M. Autopsy always teach and tell: neurodegeneration with brain iron accumulation: a case report. Indian J Pathol Microbiol. 2007;50:792–4.
Hájek M, Adamovicová M, Herynek V, Skoch A, Jírů F, Krepelová A, et al. MR relaxometry and 1 H MR spectroscopy for the determination of iron and metabolite concentrations in PKAN patients. Eur Radiol. 2005;15:1060–8.
Hayflick SJ. Unravelling the Hallervorden-Spatz syndrome: pantothenate kinase-associated neurodegeneration is the name. Curr Opin Pediatr. 2003;15:572–7.
Perry TL, Norman MG, Yong VW, Whiting S, Crichton JU, Hansen S, et al. Hallervorden-Spatz disease: cysteine accumulation and cysteine dioxygenase deficiency in the globus pallidus. Ann Neurol. 1985;18:492–9.
Hallervorden J, Spatz H. Eigenartige Erkrankung im extrapyramidalen System mit besonderer Beteiligung des Globus pallidus und der Substantia nigra. Z Ges Neurol Psychiatr. 1922;79:254–302.
Desai VS, Bindu PS, Ravishankar PN, Pal PK. Relaxation and susceptibility MRI characteristics in Hallervorden-Spatz syndrome. J Magn Reson Imaging. 2007;25:715–20.
McNeill A, Birchall D, Hayflick SJ, Gregory A, Schenk JF, Zimmerman EA, et al. T2* and FSE MRI distinguishes four subtypes of neurodegeneration with brain iron accumulation. Neurology. 2008;70:1614–9.
Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA. MRI of brain iron. Am J Roentgenol. 1986;147:103–10.
Clement F, Devos D, Moreau C, Coubes P, Destee A, Defebvre L. Neurodegeneration with brain iron accumulation: clinical, radiographic, and genetic heterogeneity and corresponding therapeutic options. Acta Neurol Belg. 2007;107:26–31.
Forni GL, Balocco M, Cremonesi L, Abbruzzese G, Parodi RC, Marchese R. Regression of symptoms after selective iron chelation therapy in a case of neurodegeneration with brain iron accumulation. Mov Disord. 2008;23:904–7.
Zorzi G, Zibordi F, Chiapparini L, Bertini E, Russo L, Piga A, et al. Iron-related MRI images in patients with pantothenate kinase-associated neurodegeneration (PKAN) treated with deferiprone: results of a phase II pilot trial. Mov Disord. 2011;26:1756–9.
Conflict of Interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fermin-Delgado, R., Roa-Sanchez, P., Speckter, H. et al. Involvement of Globus Pallidus and Midbrain Nuclei in Pantothenate Kinase-Associated Neurodegeneration. Clin Neuroradiol 23, 11–15 (2013). https://doi.org/10.1007/s00062-011-0127-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-011-0127-9