Abstract
Background and Purpose:
As potential therapies aimed at halting or slowing the decline in upper motor neuron function in patients with amyotrophic lateral sclerosis (ALS) or primary lateral sclerosis (PLS) are developed, a quantitative method for monitoring response will be necessary. Measurement of fractional anisotropy (FA) using diffusion tensor imaging (DTI) over time should parallel functional decline from upper motor neuron degeneration in these patients.
Patients and Methods:
Two patients with definite ALS were imaged at 3.0 T and FA values were obtained in the corticospinal tract every 3 months for 1 year. The FA values were compared to normal age-matched controls.
Results:
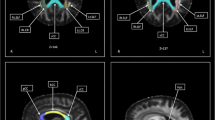
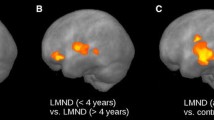
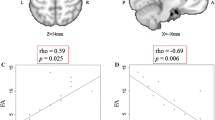
Both patients showed linear decreases in FA values over time with R2 values ranging from 0.93 to 0.99. The decline became statistically significant over the course of the study. Qualitative decreases in anisotropy were also evident on FA maps.
Conclusion:
If these trends can be validated in greater numbers of patients, DTI may serve as an objective quantitative biomarker for disease progression in patients with upper motor neuron disease.
Zusammenfassung
Hintergrund und Ziel:
Da potentielle Therapien entwickelt werden, welche auch auf die Beendigung oder Verlangsamung der Funktionsminderung der oberen Motoneuronen bei Patienten mit amyotrophischer Lateralsklerose (ALS) oder primärer Lateralsklerose (PLS) abzielen, ist eine quantitative Methode zur Reaktionskontrolle erforderlich. Die Messung der fraktionalen Anisotropie (FA) mittels Diffusions-Tensor-Bildgebung (DTI) im Zeitablauf bei diesen Patienten sollte dem funktionalen Rückgang der Degeneration der oberen Motoneuronen entsprechen.
Patienten und Methodik:
Zwei Patienten mit nachgewiesener ALS wurden einer Bildgebung mit 3,0 T unterzogen, und 1 Jahr lang wurden alle 3 Monate die FA-Werte im Tractus corticospinalis gemessen. Die FA-Werte wurden mit gesunden, altersentsprechenden Kontrollpersonen verglichen.
Ergebnisse:
Mit R2-Werten im Bereich von 0,93–0,99 wiesen beide Patienten im Zeitablauf eine lineare Abnahme der FA-Werte auf. Im Verlauf der Studie wurde die Abnahme statistisch signifikant. In den FA-Maps zeigten sich zudem qualitative Rückgänge der Anisotropie.
Schlussfolgerung:
Wenn sich diese Tendenzen bei einer größeren Anzahl von Patienten nachweisen lassen, kann die DTI bei Patienten mit oberer Motoneuronerkrankung als objektiver quantitativer Biomarker für den Krankheitsfortschritt dienen.
Similar content being viewed by others
References
Kutzke JF. Epidemiology of amyotrophic lateral sclerosis. Adv Neurol 1982;36:281–302.
Otero Siliceo E, Arriada-Mendicoa N, Balderrama J. Juvenile familial amyotrophic lateral sclerosis: four cases with long survival. Dev Med Child Neurol 1998;40:425–8.
Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases. J Neurol Sci 1994;124:96–107.
Mitsumoto H. Riluzole – what is its impact in our treatment and understanding of amyotrophic lateral sclerosis? Ann Pharmacother 1997;31:779–81.
Festoff BW, Suo Z, Citron BA. Prospects for the pharmacotherapy of amyotrophic lateral sclerosis: old strategies and new paradigms for the third millenium. CNS Drugs 2003;17:699–717.
Ulug AM, Grunewald T, Lin MT, Kamal AK, Filippi CG, Zimmerman RD, Beal MF. Diffusion tensor MR imaging in the diagnosis of primary lateral sclerosis. J Magn Reson Imaging 2004;19:34–9.
Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 1998;44:584–90.
Inder TE, Huppi PS. In vivo studies of brain development by magnetic resonance techniques. MRDD Res Rev 2000;6:59–67.
Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC. Changes in brain water diffusion during childhood. Neuroradiology 1999;41:929–34.
Nomura Y, Sakuma H, Takeda K, Tagami T, Okuda Y, Nakagawa T. Diffusion anisotropy of the human brain assessed with diffusion-weighted MR: relation with normal brain development and aging. AJNR Am J Neuroradiol 1994;15:231–8.
Sakuma H, Nomura A, Takeda T, Tagami T, Nakagawa T, Tamagawa Y, Ishii Y, Tsukamoto T. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology 1991;180:229–33.
Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Aimli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 1998;209:57–66.
Harbord MG, Finn JP, Hall-Craggs MA, Robb SA, Kendall BE, Boyd SG. Myelination patterns on magnetic resonance of children with developmental delay. Dev Med Child Neurol 1990;32:295–303.
Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, Cull TS, Conturo TE. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology 1999;212:770–84.
Ulug AM, van Zijl PCM. Orientation-independent diffusion imaging without tensor diagonalization: anisotropy definitions based on physical attributes of the diffusion ellipsoid. J Magn Reson Imaging 1999;9:804–13.
Ulug AM, Moore DF, Boyko AS, Zimmerman RD. Clinical use of diffusion tensor imaging for diseases causing neuronal and axonal damage. AJNR Am J Neuroradiol 1999;20:1044–8.
Pierpaoli P, Jezzard P, Basser PJ, Barnett A, DiChiro G. Diffusion MR imaging of the human brain. Radiology 1996;201:637–48.
Pierpaoli P, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36:893–906.
Garcia LN, Silva AV, Carrette H Jr, Favero FM, Fontes SV, Moneiro MT, Oliveira AS. Correlation between corticospinal tract degeneration through magnetic resonance imaging, and functional scale (ALSFRS) in patients with amyotrophic lateral sclerosis. Arq Neuropsiquiatr 2007;65:869–74.
Iwata NK, Aoki S, Okabe S, Arai N, Terao Y, Kwak S, Abe O, Kanazawa I, Tsuji S, Ugawa Y. Evaluation of corticospinal tracts in ALS with diffusion tensor MRI and brainstem stimulation. Neurology 2008;70:528–32.
Mitsumoto H, Ulug AM, Pullman SL, Gooch CL, Chan S, Tang MX, Mao X, Hays AP, Floyd AG, Battista V, Montes J, Hayes S, Dashaw S, Kaufmann P, Gordon PH, Hirsch J, Levin B, Rowland LP, Shungu DC. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology 2007;68:1402–10.
Sage CA, Peeters RR, Gorner A, Robberecht W, Sunaert S. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 2007;34:486–99.
ang S, Poptani H, Bilello M, Wu X, Woo JH, Elman LB, McCluskey LF, Krejza J, Melhem ER. Diffusion tensor imaging in amyotrophic lateral sclerosis: volumetric analysis of the corticospinal tract. AJNR Am J Neuroradiol 2006;27:1234–8.
Blain CR, Williams CV, Johnston C, Stanton BR, Ganesalingam J, Jarosz JM, Jones DK, Barker GJ, Williams SC, Leigh NP, Simmons A. A longitudinal study of diffusion tensor MRI in ALS. Amyotroph Lat Scleros 2007;8:348–55.
Agosta F, Rocca MA, Valsasina P, Sala S, Caputo D, Perini M, Salvi F, Prelle A, Filippi M. A longitudinal diffusion tensor MRI study of the cervical cord and brain in ALS patients. J Neurol Neurosurg Psychiatry 2009;80:53–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nickerson, J.P., Koski, C.J., Boyer, A.C. et al. Linear Longitudinal Decline in Fractional Anisotropy in Patients with Amyotrophic Lateral Sclerosis. Clin Neuroradiol 19, 129–134 (2009). https://doi.org/10.1007/s00062-009-8040-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-009-8040-1