Abstract
Background
Sepsis-induced cardiomyopathy is a well-known cause of mortality. Recent evidence has highlighted the important role of myricetin in anti-inflammation and anti-oxidative stress. However, little is known about its effect on endotoxin-induced cardiomyopathy. We examined the effect of myricetin on lipopolysaccharide (LPS)-induced cardiomyocyte injury and the underlying mechanisms in vitro.
Methods
mRNA expression of interleukin (IL)-1beta, IL-6, and tumor necrosis factor (TNF)-alpha was examined via reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Protein expression levels of NF-κB/p65, IκB, IL-1beta, IL-6, and TNF-alpha were assesses via Western blotting. Immunofluorescence (IF) was used to determine the nuclear translocation of p65. Commercial kits were employed to detect the level of oxidative markers and to quantify NF-κB/p65 both in the cytoplasm and the nucleus. Finally, terminal deoxy-nucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was performed to evaluate the apoptosis of H9c2 cardiomyocytes.
Results
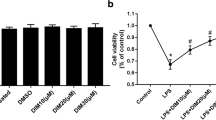
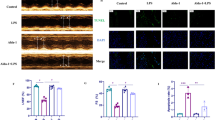
The results showed that myricetin blunted the overexpression of IL-1beta, IL-6, and TNF-alpha markedly by inhibiting the NF-κB/P65 signaling pathway. Furthermore, myricetin treatment led to the downregulation of reactive oxygen species (ROS) accompanied by increased expression of superoxide dismutase and glutathione peroxidase. TUNEL-positive nuclei were rarely detected following myricetin treatment.
Conclusion
Our findings suggest that myricetin is a valuable protective agent against endotoxin-induced early inflammatory responses in H9c2 cardiomyocytes, which involves regulation of ROS and the IκB/NF-κb signaling pathway.
Zusammenfassung
Hintergrund
Die sepsisinduzierte Kardiomyopathie gehört zu den bekannten Todesursachen. Aktuelle Daten betonen die Bedeutung von Myricetin bei antientzündlichen Prozessen und antioxidativem Stress. Jedoch gibt es nur wenige Erkenntnisse zu seiner Wirkung auf die endotoxininduzierte Kardiomyopathie. Wir untersuchten nun die Wirkung von Myricetin auf lipopolysaccharid(LPS)-induzierte Kardiomyozytenläsionen und die zugrunde liegenden Mechanismen in vitro.
Methoden
Die mRNA-Expression von Interleukin(IL)-1beta, IL-6 und Tumornekrosefaktor(TNF)-alpha wurde mittels RT-qPCR („reverse transcription-quantitative polymerase chain reaction“) untersucht. Außerdem wurden die Proteinexpressionsspiegel von NF-κB/p65, IκB, IL-1beta, IL-6 und TNF-alpha mittels Westernblottest gemessen. Die Immunfluoreszenz (IF) wurde eingesetzt, um die nukleäre Translokation von p65 zu bestimmen. Kommerziell erhältliche Tests wurden verwendet, um die Werte für oxidative Marker zu ermitteln und NF-κB/p65 sowohl im Zytoplasma als auch im Nukleus zu quantifizieren. Schließlich wurde die TUNEL-Methode („terminal deoxy-nucleotidyl transferase-mediated dUTP nick end labeling“) angewendet, um die Apopotose von H9c2-Kardiomyozyten zu untersuchen.
Ergebnisse
Die Ergebnisse zeigten, das Myricetin die Überexpression von IL-1beta, IL-6 und TNF-alpha deutlich durch Inhibition des NF-κB/P65-Signalwegs abschwächte. Darüber hinaus führte die Behandlung mit Myricetin zur Herunterregulierung reaktiver Oxygenspezies (ROS), begleitet von einer erhöhten Expression der Superoxiddismutase und der Glutathionperoxidase. TUNEL-positive Nuclei wurden nach Myricetinbehandlung nur selten entdeckt.
Schlussfolgerung
Den vorliegenden Ergebnissen zufolge ist Myricetin eine nützliche protektive Substanz gegen endotoxininduzierte frühe entzündliche Reaktionen bei H9c2-Kardiomyozyten, wozu auch die Regulation von ROS und des IκB/NFκb-Signalwegs gehört.








Similar content being viewed by others
References
Singer M, Deutschman CS, Seymour CW et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810
Vincent JL, Marshall JC, Namendys-Silva SA et al (2014) Assessment of the worldwide burden of critical illness: the intensive care over nations (icon) audit. Lancet Respir Med 2:380–386
Vieillard-Baron A (2011) Septic cardiomyopathy. Ann Intensive Care 1:6
Marchant DJ, Boyd JH, Lin DC et al (2012) Inflammation in myocardial diseases. Circ Res 110:126–144
Sato R, Nasu M (2015) A review of sepsis-induced cardiomyopathy. J Intensive Care 3:48
Ma H, Wang X, Ha T et al (2016) Microrna-125b prevents cardiac dysfunction in polymicrobial sepsis by targeting traf6-mediated nuclear factor kappab activation and p53-mediated apoptotic signaling. J Infect Dis 214:1773–1783
Yang P, Han Y, Gui L et al (2013) Gastrodin attenuation of the inflammatory response in h9c2 cardiomyocytes involves inhibition of nf-kappab and mapks activation via the phosphatidylinositol 3‑kinase signaling. Biochem Pharmacol 85:1124–1133
Planavila A, Rodriguez-Calvo R, Jove M et al (2005) Peroxisome proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc Res 65:832–841
Kumar A, Thota V, Dee L et al (1996) Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 183:949–958
Turdi S, Han X, Huff AF et al (2012) Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced myocardial contractile dysfunction: role of autophagy. Free Radic Biol Med 53:1327–1338
Yao X, Carlson D, Sun Y et al (2015) Mitochondrial ros induces cardiac inflammation via a pathway through mtdna damage in a pneumonia-related sepsis model. PLOS ONE 10:e0139416
Suliman HB, Welty-Wolf KE, Carraway M et al (2004) Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res 64:279–288
Sperandeo P, Martorana AM, Polissi A (2016) Lipopolysaccharide biogenesis and transport at the outer membrane of gram-negative bacteria. Biochim Biophys Acta. doi:10.1016/j.bbalip.2016.10.006
Semwal DK, Semwal RB, Combrinck S et al (2016) A dietary molecule with diverse biological activities. Nutrients 8:90
Cho BO, Yin HH, Park SH et al (2016) Anti-inflammatory activity of myricetin from diospyros lotus through suppression of nf-kappab and stat1 activation and nrf2-mediated ho-1 induction in lipopolysaccharide-stimulated raw264.7 macrophages. Biosci Biotechnol Biochem 80:1520–1530
Ullah R, Nadeem M, Khalique A et al (2016) Nutritional and therapeutic perspectives of chia (salvia hispanica l.): a review. J Food Sci Technol 53:1750–1758
Ko SY et al (2012) Myricetin suppresses lps-induced mmp expression in human gingival fibroblasts and inhibits osteoclastogenesis by downregulating nfatc1 in rankl-induced raw 264.7 cells. Arch Oral Biol 57:1623–1632
Niu J, Wang K, Graham S et al (2011) Mcp-1-induced protein attenuates endotoxin-induced myocardial dysfunction by suppressing cardiac nf-small ka, cyrillicb activation via inhibition of ismall ka, cyrillicb kinase activation. J Mol Cell Cardiol 51:177–186
Planavila A, Redondo-Angulo I, Ribas F et al (2015) Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res 106:19–31
Alhamdi Y, Abrams ST, Cheng Z et al (2015) Circulating histones are major mediators of cardiac injury in patients with sepsis. Crit Care Med 43:2094–2103
Rudiger A, Singer M (2007) Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35:1599–1608
Mayr FB, Yende S, Angus DC (2014) Epidemiology of severe sepsis. Virulence 5:4–11
Fu RH, Liu SP, Chu CL et al (2013) Myricetin attenuates lipopolysaccharide-stimulated activation of mouse bone marrow-derived dendritic cells through suppression of ikk/nf-kappab and mapk signalling pathways. J Sci Food Agric 93:76–84
Wu S, Yue Y, Peng A et al (2016) Myricetin ameliorates brain injury and neurological deficits via nrf2 activation after experimental stroke in middle-aged rats. Food Funct 7:2624–2634
Qin S, Chen J, Tanigawa S et al (2013) Microarray and pathway analysis highlight nrf2/are-mediated expression profiling by polyphenolic myricetin. Mol Nutr Food Res 57:435–446
Acknowledgments
This work was supported by Grants from Doctoral Scientific Research Foundation of Hubei University of Science and Technology (BK1417).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Chen and B. Fan declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chen, S., Fan, B. Myricetin protects cardiomyocytes from LPS-induced injury. Herz 43, 265–274 (2018). https://doi.org/10.1007/s00059-017-4556-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-017-4556-3