Abstract
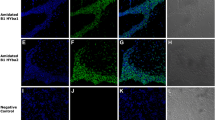
Two new antimicrobial peptides belonging to brevinin 1 (B1) and brevinin 2 (B2) families were identified from the skin secretion of Indosylvirana aurantiaca, an endemic frog of Western Ghats, India, through shotgun cloning. Antibacterial, antibiofilm and cytotoxic effects of these peptides were evaluated and compared with their C-terminally amidated forms. Both the amidated peptides (B1-NH2 and B2-NH2) showed significantly enhanced broad-spectrum activities against Gram-positive and Gram-negative bacteria and the latter was found to have potent biofilm inhibition properties against Klebsiella pneumoniae over other peptides. Both forms of B2 were less cytotoxic against human red blood cells (hRBC) and peripheral blood mononuclear cell (PBMC). Hence, B2 and its amidated analog have good therapeutic value and these peptides could be considered as potential lead molecules for further development.




Similar content being viewed by others
References
Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S (2004) Bacterial persistence as a phenotypic switch. Science 305:1622–1625
Bobone S, Bocchinfuso G, Park Y, Palleschi A, Hahm KS, Stella L (2013) The importance of being kinked: role of Pro residues in the selectivity of the helical antimicrobial peptide P5. J Pept Sci 19:758–769
Cady NC, McKean KA, Behnke J, Kubec R, Mosier AP, Kasper SH, Burz DS, Musah RA (2012) Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS One 7:384–392
Chen T, Li L, Zhou M, Rao P, Walker B, Shaw C (2006) Amphibian skin peptides and their corresponding cDNAs from single lyophilized secretion samples: identification of novel brevinins from three species of Chinese frogs. Peptides 27:42–48
Clinical and Laboratory Standards Institute (2012) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, CLSI document M07-A9, 9th edn. Clinical and Laboratory Standard Institute, Wayne, PA
Combet C, Blanchet C, Geourjon C, Deleage G (2000) NPS@: network protein sequence analysis. Trends Biochem Sci 25:147–150
Conlon JM, Al-Kharrge R, Ahmed E, Raza H, Galadari S, Condamine E (2007) Effect of aminoisobutyric acid (Aib) substitutions on the antimicrobial and cytolytic activities of the frog skin peptide, temporin-1DRa. Peptides 28:2075–2080
da Silva A, De Souza BM, dos Santos Cabrera MP, Dias NB, Gomes PC, Neto JR, Stabeli RG, Palma MS (2014) The effects of the C-terminal amidation of mastoparans on their biological actions and interactions with membrane-mimetic systems. Biochim Biophys Acta Biomembr 1838:2357–2368
de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock RE (2013) Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16:580–589
Dempsey CE, Bazzo R, Harvey S, Syperek I, Boheim G, Campbell ID (1991) Contribution of proline-14 to the structure and actions of melittin. FEBS Lett 281:240–244
Giangaspero A, Sandr L, Tossi A (2001) Amphipathic α helical antimicrobial peptides. FEBS J 268:5589–5600
Hancock RE (2001) Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1:156–164
Ilić N, Novković M, Guida F, Xhindoli D, Benincasa M, Tossi A, Juretić D (2013) Selective antimicrobial activity and mode of action of adepantins, glycine-rich peptide antibiotics based on anuran antimicrobial peptide sequences. Biochim Biophys Acta Biomembr 1828:1004–1012
Kim JY, Park SC, Yoon MY, Hahm KS, Park Y (2011) C-terminal amidation of PMAP-23: translocation to the inner membrane of Gram-negative bacteria. Amino Acids 40:183–195
König E, Bininda-Emonds OR, Shaw C (2015) The diversity and evolution of anuran skin peptides. Peptides 63:96–117
Kumar VT, Holthausen D, Jacob J, George S (2015) Host defense peptides from Asian frogs as potential clinical therapies. Antibiotics 4:136–159
Kumar TV, Asha R, Shyla G, George S (2017) Identification and characterization of novel host defense peptides from the skin secretion of the fungoid frog, Hydrophylaxbahuvistara (Anura: Ranidae). Chem Biol Drug Des 92(2):1409–1418
Laverty G, Gorman SP, Gilmore B (2011) The potential of antimicrobial peptides as biocides. Int J Mol Sci 12:6566–6596
Lee DL, Hodges RS (2003) Structure–activity relationships of de novo designed cyclic antimicrobial peptides based on gramicidin S. Pept Sci 71:28–48
Matsuzaki K (1999) Why and how are peptide–lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta Biomembr 1462:1–10
Monroe D (2007) Looking for chinks in the armor of bacterial biofilms. PLoS Biol 5:e307
Oliver LA, Prendini E, Kraus F, Raxworthy CJ (2015) Systematics and biogeography of the Hylarana frog (Anura: Ranidae) radiation across tropical Australasia, Southeast Asia, and Africa. Mol Phylogenet Evol 90:176–192
Park SC, Park Y, Hahm KS (2011) The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci 12:5971–5992
Savelyeva A, Ghavami S, Davoodpour P, Asoode A, Łos MJ (2014) An overview of brevinin superfamily: structure, function and clinical perspectives. Adv Exp Med Biol 818:197–212
Shahmiri M, Enciso M, Mechler A (2015) Controls and constrains of the membrane disrupting action of Aurein 1.2. Sci Rep 5:16378
Shalev DE, Mor A, Kustanovich I (2002) Structural consequences of carboxyamidation of dermaseptin S3. Biochemistry 41:7312–7317
Strandberg E, Tiltak D, Ieronimo M, Kanithasen N, Wadhwani P, Ulrich AS (2007) Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic α-helical peptides. Pure Appl Chem 79:717–728
Thomas P, Kumar TV, Reshmy V, Kumar KS, George S (2012) A mini review on the antimicrobial peptides isolated from the genus Hylarana (Amphibia: Anura) with a proposed nomenclature for amphibian skin peptides. Mol Biol Rep 39:6943–6947
Van Dijk A, Molhoek EM, Veldhuizen EJ, Tjeerdsma-van Bokhoven JL, Wagendorp E, Bikker F, Haagsman HP (2009) Identification of chicken cathelicidin-2 core elements involved in antibacterial and immunomodulatory activities. Mol Immunol 46:2465–2473
Yang ST, Lee JY, Kim HJ, Eu Y, Shin SY, Hahm KS, Kim JI (2006) Contribution of a central proline in model amphipathic α-helical peptides to self-association, interaction with phospholipids, and antimicrobial mode of action. FEBS J 273:4040–4054
Zelezetsky I, Tossi A (2006) Alpha-helical antimicrobial peptides-using a sequence template to guide structure-activity relationship studies. Biochim Biophy Acta Biomembr 1758(9):1436–1449
Zhang SK, Song JW, Gong F, Li SB, Chang HY, Xie HM, Gao HW, Tan HW, Tan YX, Ji SP (2016) Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci Rep 6:27394
Acknowledgements
This work was financially supported by Kerala State Council for Science Technology and Environment under Verghese Kurien Young Scientist Fellowship Scheme (Order no. 717/2014/KSCSTE). SHG is thankful to The Director, Rajiv Gandhi Centre for Biotechnology, for providing facilities for conducting the work properly. The authors are also grateful to Kerala Forest Department for permitting sample collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interests.
Ethical approval
The protocols of the assays with human samples were approved by the human ethics committee of Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala.
Additional information
Communicated by Günther Raspotnig.
Rights and permissions
About this article
Cite this article
Shyla, G., Vineethkumar, T.V., Arun, V. et al. Functional characterization of two novel peptides and their analogs identified from the skin secretion of Indosylvirana aurantiaca, an endemic frog species of Western Ghats, India. Chemoecology 29, 179–187 (2019). https://doi.org/10.1007/s00049-019-00287-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-019-00287-z