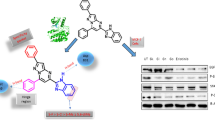
Abstract
A [2+2]-cycloaddition of bis-isatin Schiff bases 5a–b and 7 with activated aryloxyacetic acid derivatives 8a–d afforded bis-spiroisatino β-lactams with aliphatic and aromatic spacers. The structures of the synthesized 2-oxindoles and spirooxindoles were determined based on Fourier-transform infrared spectroscopy, proton-1 and carbon-13 nuclear magnetic resonance spectroscopies and CHN analysis. Our interest in these bis-Schiff bases and bis-spiroisatino β-lactams is for their potential anticancer capabilities. In vitro bioactivity testing against the cervical adenocarcinoma (HeLa) and breast cancer (MCF-7) cell lines as well as noncancerous NIH/3T3 fibroblast cell was investigated applying the MTT assay. Bis-isatin derivatives 5a, 5b, 9h, 10a, and 10b showed promising antiproliferative activity toward these two cancer cell lines. Two of the bis-isatin Schiff bases, 5a and 5b, displayed IC50 values less than that of the clinically-used anticancer agent cisplatin towards both the MCF-7 and HeLa cells, while several of the bis-isatin β-lactams showed similar bioactivity to that of cisplatin. All of the oxindoles and spirooxindoles displayed a selective anticancer effect except 10b. To study the possible mechanism for this bioactivity, DNA and BSA binding analyses were performed using fluorescence and UV–visible spectroscopic techniques. The isatin adducts displayed excellent interaction propensity to CT-DNA as well as BSA. Molecular docking investigation carried out on DNA and BSA with promising molecules to show the possible mechanism of cytotoxicity activities.





































Similar content being viewed by others
References
Mahmoud YK, Abdelrazek HM. Cancer: thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed Pharmacother. 2019;115:108783.
Meeran MN, Hussain A. Synthesis, characterization and DPPH scavenging assay of isatin related spiroheterocyclic compounds. Indian J Pharm Sci. 2017;79:641–5.
Yu B, Yu D-Q, Liu H-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur J Med Chem. 2015;97:673–98.
Singh GS, Desta ZY. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem Rev. 2012;112:6104–55.
Wang J, Huang D, Wang K-H, Peng X, Su Y, Hu Y, et al. Tin powder-promoted one-pot synthesis of 3-spiro-fused or 3, 3′-disubstituted 2-oxindoles. Org Biomol Chem. 2016;14:9533–42.
El-Sharief AMS, Ammar YA, Belal A, El-Sharief MAS, Mohamed YA, Mehany AB, et al. Design, synthesis, molecular docking and biological activity evaluation of some novel indole derivatives as potent anticancer active agents and apoptosis inducers. Bioorg Chem. 2019;85:399–412.
Yang R-Y, Sun J, Sun Q, Yan C-G. Selective construction of polycyclic spirooxindoles via a Cu (OTf) 2/HOTf-catalyzed domino reaction of o-arylalkynylacetophenones and 3-phenacylideneoxindoles. Org Biomol Chem. 2017;15:6353–7.
Wei Q, Gong L-Z. Organocatalytic asymmetric formal [4+2] cycloaddition for the synthesis of spiro [4-cyclohexanone-1, 3′-oxindoline] derivatives in high optical purity. Org lett. 2010;12:1008–11.
Han J-L, Chang C-H. An asymmetric assembly of spirooxindole dihydropyranones through a direct enantioselective organocatalytic vinylogous aldol-cyclization cascade reaction of 3-alkylidene oxindoles with isatins. Chem Commun. 2016;52:2322–5.
Viegas-Junior C, Danuello A, da Silva Bolzani V, Barreiro EJ, Fraga CAM. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem. 2007;14:1829–52.
Nepali K, Sharma S, Sharma M, Bedi P, Dhar K. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem. 2014;77:422–87.
Hulsman N, Medema JP, Bos C, Jongejan A, Leurs R, Smit MJ, et al. Chemical insights in the concept of hybrid drugs: the antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J Med Chem. 2007;50:2424–31.
Mishra S, Singh P. Hybrid molecules: the privileged scaffolds for various pharmaceuticals. Eur J Med Chem. 2016;124:500–36.
Zhang L, Xu Z. Coumarin-containing hybrids and their anticancer activities. Eur J Med Chem. 2019;18:111587–606.
Gao F, Wang T, Gao M, Zhang X, Liu Z, Zhao S, et al. Benzofuran-isatin-imine hybrids tethered via different length alkyl linkers: Design, synthesis and in vitro evaluation of anti-tubercular and anti-bacterial activities as well as cytotoxicity. Eur J Med Chem. 2019;165:323–31.
Riazimontazer E, Sadeghpour H, Nadri H, Sakhteman A, Küçükkılınç TT, Miri R, et al. Design, synthesis and biological activity of novel tacrine-isatin Schiff base hybrid derivatives. Bioorg Chem. 2019;89:103006.
Alpaslan G, Boyacioglu B, Demir N, Tümer Y, Yapar G, Yıldırım N, et al. Synthesis, characterization, biological activity and theoretical studies of a 2-amino-6-methoxybenzothiazole-based fluorescent Schiff base. J Mol Struct. 2019;1180:170–8.
Pervez H, Ahmad M, Zaib S, Yaqub M, Naseer MM, Iqbal J. Synthesis, cytotoxic and urease inhibitory activities of some novel isatin-derived bis-Schiff bases and their copper (II) complexes. Med Chem Comm. 2016;7:914–23.
Muğlu H, Çavuş MS, Bakır T, Yakan H. Synthesis, characterization, quantum chemical calculations and antioxidant activity of new bis-isatin carbohydrazone and thiocarbohydrazone derivatives. J Mol Struct. 2019;1196:819–27.
Avaji PG, Kumar CV, Patil SA, Shivananda K, Nagaraju C. Synthesis, spectral characterization, in-vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bis hydrazone. Eur J Med Chem. 2009;44:3552–9.
Khan KM, Khan M, Ali M, Taha M, Rasheed S, Perveen S, et al. Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg Med Chem. 2009;17:7795–801.
Fisher JF, Mobashery S. The β-Lactam (Azetidin-2-one) as a privileged ring in medicinal chemistry. In: Privileged scaffolds in medicinal chemistry: Design, Synthesis, Evaluation. London: Royal Society of Chemistry; 2015. p. 64–97.
Ranjbari S, Behzadi M, Sepehri S, Aseman MD, Jarrahpour A, Mohkam M, et al. Investigations of antiproliferative and antioxidant activity of β-lactam morpholino-1, 3, 5-triazine hybrids. Bioorg Med Chem. 2020;28:115408.
Borazjani N, Behzadi M, Dadkhah Aseman M, Jarrahpour A, Rad JA, Kianpour S, et al. Cytotoxicity, anticancer, and antioxidant properties of mono and bis-naphthalimido β-lactam conjugates. Med Chem Res. 2020;29:1–21.
Bashiri M, Jarrahpour A, Rastegari B, Iraji A, Irajie C, Amirghofran Z, et al. Synthesis and evaluation of biological activities of tripodal imines and β‑lactams attached to the 1, 3, 5‑triazine nucleus. Monatsh Chem. 2020;151:821–35.
Jarrahpour A, Khalili D. Synthesis of some mono-and bis-spiro-β-lactams of benzylisatin. Tetrahedron Lett. 2007;48:7140–3.
Jarrahpour A, Khalili D, De Clercq E, Salmi C, Brunel J. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules. 2007;12:1720–30.
Shekouhy M, Khalafi-Nezhad A. Polyethylene glycol-bonded 1,8-diazabicyclo [5.4. 0] undec-7-ene (PEG–DBU) as a surfactant-combined base catalyst for the application of nucleosides as reagents in multi-component syntheses of 8-substituted pyrido [2, 3-d] pyrimidine-6-carbonitriles in water. Green Chem. 2015;17:4815–29.
Panda SS, Jain N, Jehan N, Bhagat S, Jain SC. An eco-friendly synthesis of some novel symmetrical bis spiro-indoles. Phosphorus Sulfur. 2012;187:101–11.
Reichmann M, Rice S, Thomas C, Doty P. A further examination of the molecular weight and size of desoxypentose nucleic acid. J Am Chem Soc. 1954;76:3047–53.
Staudinger H. Zur kenntniss der Ketene. diphenylketen. Liebigs Ann. 1907;356:51–123.
Liu Z-C, Wang B-D, Yang Z-Y, Li Y, Qin D-D, Li T-R. Synthesis, crystal structure, DNA interaction and antioxidant activities of two novel water-soluble Cu2+ complexes derivated from 2-oxo-quinoline-3-carbaldehyde Schiff-bases. Eur J Med Chem. 2009;44:4477–84.
Krishnamoorthy P, Sathyadevi P, Cowley AH, Butorac RR, Dharmaraj N. Evaluation of DNA binding, DNA cleavage, protein binding and in vitro cytotoxic activities of bivalent transition metal hydrazone complexes. Eur J Med Chem. 2011;46:3376–87.
Manikandamathavan VM, Parameswari RP, Weyhermüller T, Vasanthi HR, Nair BU. Cytotoxic copper (II) mixed ligand complexes: crystal structure and DNA cleavage activity. Eur J Med Chem. 2011;46:4537–47.
Mei WJ, Liu J, Zheng KC, Lin LJ, Chao H, Li AX. et al. Experimental and theoretical study on DNA-binding and photocleavage properties of chiral complexes Δ-and Λ-[Ru (bpy) 2 L](L = o-hpip, m-hpip and p-hpip). Dalton Trans.2003;7:1352–9.
Pratviel G, Bernadou J, Meunier B. DNA and RNA cleavage by metal complexes. Adv Inorg Chem. 1998;45:251–312.
Shahabadi N, Kashanian S, Khosravi M, Mahdavi M. Multispectroscopic DNA interaction studies of a water-soluble nickel (II) complex containing different dinitrogen aromatic ligands. Transit Met Chem. 2010;35:699–705.
Pyle A, Rehmann J, Meshoyrer R, Kumar C, Turro N, Barton JK. Mixed-ligand complexes of ruthenium (II): factors governing binding to DNA. J Am Chem Soc. 1989;111:3051–8.
Ghosh K, Kumar P, Tyagi N, Singh UP, Aggarwal V, Baratto MC. Synthesis and reactivity studies on new copper (II) complexes: DNA binding, generation of phenoxyl radical, SOD and nuclease activities. Eur J Med Chem. 2010;45:3770–9.
Rad JA, Jarrahpour A, Aseman MD, Nabavizadeh M, Pournejati R, Karbalaei‐Heidari HR, et al. Design, synthesis, DNA binding, cytotoxicity, and molecular docking studies of amonafide‐linked β‐lactam. ChemistrySelect. 2019;4:2741–6.
Cory M, McKee DD, Kagan J, Henry D, Miller JA. Design, synthesis, and DNA binding properties of bifunctional intercalators. Comparison of polymethylene and diphenyl ether chains connecting phenanthridine. J Am Chem Soc. 1985;107:2528–36.
Dhar S, Nethaji M, Chakravarty AR. Effect of charge transfer bands on the photo-induced DNA cleavage activity of [1-(2-thiazolylazo)-2-naphtholato] copper (II) complexes. J Inorg Biochem. 2005;99:805–12.
Li W-Y, Xu J-G, Guo X-Q, Zhu Q-Z, Zhao Y-B. Study on the interaction between rivanol and DNA and its application to DNA assay. Spectrochim Acta A. 1997;53:781–7.
Suh D, Chaires JB. Criteria for the mode of binding of DNA binding agents. Bioorg Med Chem. 1995;3:723–8.
Lakowicz JR. Principles of fluorescence spectroscopy. New York: Springer Science & Business Media; 2013.
Arjmand F, Mohani B, Ahmad S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu (II) complex. Eur J Med Chem. 2005;40:1103–10.
Kelly JM, Tossi AB, McConnell DJ, OhUigin C. A study of the interactions of some polypyridylruthenium (II) complexes with DNA using fluorescence spectroscopy, topoisomerisation and thermal denaturation. Nucleic Acids Res. 1985;13:6017–34.
Bhat SS, Kumbhar AS, Lönnecke P, Hey-Hawkins E. Self-Association of ruthenium (II) polypyridyl complexes and their interactions with calf thymus DNA. Inorg Chem. 2010;49:4843–53.
Mandegani Z, Asadi Z, Asadi M, Karbalaei-Heidari HR, Rastegari B. Synthesis, characterization, DNA binding, cleavage activity, cytotoxicity and molecular docking of new nano water-soluble [M (5-CH 2 PPh 3-3, 4-salpyr)](ClO 4) 2 (M = Ni, Zn) complexes. Dalton Trans. 2016;45:6592–611.
Shahabadi N, Kashanian S, Darabi F. In vitro study of DNA interaction with a water-soluble dinitrogen Schiff base. DNA Cell Biol. 2009;28:589–96.
Feng X-Z, Lin Z, Yang L-J, Wang C, Bai C-l. Investigation of the interaction between acridine orange and bovine serum albumin. Talanta. 1998;47:1223–9.
Qin P, Liu R, Pan X, Fang X, Mou Y. Impact of carbon chain length on binding of perfluoroalkyl acids to bovine serum albumin determined by spectroscopic methods. J Agric Food Chem. 2010;58:5561–7.
Roy AS, Tripathy DR, Chatterjee A, Dasgupta S. A spectroscopic study of the interaction of the antioxidant naringin with bovine serum albumin. J Biophys Chem. 2010;1:141.
Zhang L, Cai Q-Y, Cai Z-X, Fang Y, Zheng C-S, Wang L-L, et al. Interactions of bovine serum albumin with anti-cancer compounds using a Proteon XPR36 array biosensor and molecular docking. Molecules. 2016;21:1706.
Raja DS, Bhuvanesh NS, Natarajan K. A novel water soluble ligand bridged cobalt (II) coordination polymer of 2-oxo-1,2-dihydroquinoline-3-carbaldehyde (isonicotinic) hydrazone: evaluation of the DNA binding, protein interaction, radical scavenging and anticancer activity. Dalton Trans. 2012;41:4365–77.
Miller J. Photoluminescence and chemiluminescence methods of drug analysis. J Pharm Biomed. 1983;1:525–35.
Raja DS, Ramachandran E, Bhuvanesh NS, Natarajan K. Synthesis, structure and in vitro pharmacological evaluation of a novel 2-oxo-1,2-dihydroquinoline-3-carbaldehyde (2′-methylbenzoyl) hydrazone bridged copper (II) coordination polymer. Eur J Med Chem. 2013;64:148–59.
Ramachandran E, Thomas SP, Poornima P, Kalaivani P, Prabhakaran R, Padma VV, et al. Evaluation of DNA binding, antioxidant and cytotoxic activity of mononuclear Co (III) complexes of 2-oxo-1,2-dihydrobenzo[h] quinoline-3-carbaldehyde thiosemicarbazones. Eur J Med Chem. 2012;50:405–15.
Gupta RK, Pandey R, Sharma G, Prasad R, Koch B, Srikrishna S, et al. DNA binding and anti-cancer activity of redox-active heteroleptic piano-stool Ru (II), Rh (III), and Ir (III) complexes containing 4-(2-methoxypyridyl) phenyldipyrromethene. Inorg Chem. 2013;52:3687–98.
He Y, Wang Y, Tang L, Liu H, Chen W, Zheng Z, et al. Binding of puerarin to human serum albumin: a spectroscopic analysis and molecular docking. J Fluoresc. 2008;18:433–42.
Divsalar A, Bagheri MJ, Saboury AA, Mansoori-Torshizi H, Amani M. Investigation on the interaction of newly designed anticancer Pd (II) complexes with different aliphatic tails and human serum albumin. J Phys Chem B. 2009;113:14035–42.
Ross PD, Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochem. 1981;20:3096–102.
Zarei L, Asadi Z, Dusek M, Eigner V. Homodinuclear Ni (II) and Cu (II) Schiff base complexes derived from O-vanillin with a pyrazole bridge: Preparation, crystal structures, DNA and protein (BSA) binding, DNA cleavage, molecular docking and cytotoxicity study. J Photochem A. 2019;374:145–60.
Rohs R, Bloch I, Sklenar H, Shakked Z. Molecular flexibility in ab initio drug docking to DNA: binding-site and binding-mode transitions in all-atom Monte Carlo simulations. Nucleic Acids Res. 2005;33:7048–57.
Tanzadehpanah H, Mahaki H, Moghadam NH, Salehzadeh S, Rajabi O, Najafi R, et al. Binding site identification of anticancer drug gefitinib to HSA and DNA in the presence of five different probes. J Biomol Struct Dyn. 2019;37:823–36.
Majorek KA, Porebski PJ, Dayal A, Zimmerman MD, Jablonska K, Stewart AJ, et al. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol Immunol. 2012;52:174–82.
Acknowledgements
The authors would like to thank the Shiraz University Research Council for financial support (Grant no. 97-GR-SC-23).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bashiri, M., Jarrahpour, A., Nabavizadeh, S.M. et al. Potent antiproliferative active agents: novel bis Schiff bases and bis spiro β-lactams bearing isatin tethered with butylene and phenylene as spacer and DNA/BSA binding behavior as well as studying molecular docking. Med Chem Res 30, 258–284 (2021). https://doi.org/10.1007/s00044-020-02659-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02659-5