Abstract
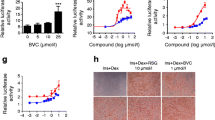
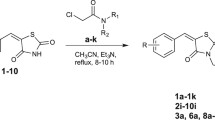
Peroxisome proliferator-activated receptors (PPARs) agonists contribute to the regulation of glucose, lipid, and cholesterol metabolism and have emerged as key targets to treat metabolic syndrome. In our previous study, the natural compound bavachinin was found to have pan-PPAR agonist activity. In this study, five isoflavones, three isoflavanones, and five scaffold-hopping analogues of bavachinin were designed, synthesised, and evaluated through reporter gene assays for pan-PPAR agonist activity. The analogue 2-(4-hydroxyphenyl)-6-isopentenyl-7-methoxy-2,3-dihydroquinolin-4(1H)-one (21) was identified as a pan-PPAR agonist, exhibiting substantially higher PPAR α/β agonist activity and equal PPAR-γ agonist activity than does bavachinin.





Similar content being viewed by others
References
Beltrán-Sánchez H, Harhay MO, Harhay MM, Mcelligott S (2013) Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol 62:697–703
Biegasiewicz KF, Gordon JS, Rodriguez DA, Priefer R (2014) Development of a general approach to the synthesis of a library of isoflavonoid derivatives. Tetrahedron Lett 55:5210–5212
Cairns WJ (2004) Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol 122:971–983
Del Bas JMD, Laos S, Caimari A, Crescenti A, Arola L (2012) Detection of bioavailable peroxisome proliferator-activated receptor gamma modulators by a cell-based luciferase reporter system. Anal Biochem 427:187–189
Du GX, Feng L, Yang Z, Shi J, Huang C, Guo F, Li B, Zhu W, Li Y (2015) Separation and peroxisome proliferator-activated receptor-γ agonist activity evaluation of synthetic racemic bavachinin enantiomers. Bioorg Med Chem Lett 25:2579–2583
Du GX, Zhao YY, Feng L, Yang Z, Shi J, Huang C, Li B, Guo F, Zhu W, Li Y (2017) Design, synthesis, and structure–activity relationships of bavachinin analogues as peroxisome proliferator-activated receptor γ agonists. ChemMedChem 12:183–193
Farmer JL, Hunter HN, Organ MG (2012) Regioselective cross-coupling of allylboronic acid pinacol ester derivatives with aryl halides via Pd-PEPPSI-IPent. J Am Chem Soc 134:17470–174703
Feng L, Luo H, Xu ZJ, Yang Z, Du G, Zhang Y, Yu L, Hu K, Zhu W, Tong Q, Chen K, Guo F, Huang C, Li Y (2016) Bavachinin, as a novel natural pan-PPAR agonist, exhibits unique synergistic effects with synthetic PPAR-γ and PPAR-α agonists on carbohydrate and lipid metabolism in db/db and diet-induced obese mice. Diabetologia 59:1276–1286
Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodríguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D (2003) Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425:90–93
Gim HJ, Li H, Jung SR, Park YJ, Ryu JH, Chung KH, Jeon R (2014) Design and synthesis of azaisoflavone analogs as phytoestrogen mimetics. Eur J Med Chem 85:107–118
Gisch N, Balzarini J, Meier C (2007) Enzymatically activated cycloSal-d4T-monophosphates: the third generation of cycloSal-pronucleotides. J Med Chem 50:1658–1667
Grundy SM (2016) Metabolic syndrome update. Trends Cardiovasc Med 26:364–373
Jin Z, Lin H, Srinivasan S, Nwachukwu JC, Bruno N, Griffin PR, Nettles KW, Kamenecka TM (2017) Synthesis of novel steroidal agonists, partial agonists, and antagonists for the glucocorticoid receptor. Bioorg Med Chem 27:347–353
Liu Z, Zhang H, Ye N, Zhang J, Wu Q, Sun P, Li L, Zhen X, Zhang A (2010) Synthesis of dihydrofuroaporphine derivatives: identification of a potent and selective serotonin 5-HT 1A receptor agonist. J Med Chem 53:1319–1328
Ma S, Huang Y, Zhao Y, Du G, Feng L, Huang C, Li Y, Guo F (2016) Prenylflavone derivatives from the seeds of Psoralea corylifolia exhibited PPAR-γ agonist activity. Phytochem Lett 16:213–218
Malan-Müller S, Kilian S, Van LL, Bardien S, Asmal L, Warnich L, Emsley RA, Hemmings SM, Seedat S (2016) A systematic review of genetic variants associated with metabolic syndrome in patients with schizophrenia. Schizophr Res 170:1–17
Matin A, Doddareddy MR, Gavande N, Nammi S, Groundwater PW, Roubin RH, Hibbs DE (2013) The discovery of novel isoflavone pan peroxisome proliferator-activated receptor agonists. Bioorg Med Chem 21:766–778
Mutai P, Pavadai E, Wiid I, Ngwane A, Baker B, Chibale K (2015) Synthesis, antimycobacterial evaluation and pharmacophore modeling of analogues of the natural product formononetin. Bioorg Med Chem Lett 25:2510–2513
Pourcet B, Fruchart JC, Staels B, Glineur C (2006) Selective PPAR modulators, dual and pan PPAR agonists: multimodal drugs for the treatment of type-2 diabetes and atherosclerosis. Expert Opin Emerg Drugs 11:379–401
Wang GL, Chen XL, Deng YY, Li Z, Xu X (2015) Synthesis and nematicidal activities of 1,2,3-Benzotriazin-4-one derivatives against Meloidogyne incognita. J Agric Food Chem 63:6883–6889
Wang XB, Xu DL (2017) A radical cascade enabling collective syntheses of natural products. Chem 2:803–816
Wehle S, Espargarό A, Sabaté R, Decker M (2016) Investigation into the stability and reactivity of the pentacyclic alkaloid dehydroevodiamine and the benz-analog there of. Tetrahedron 72:2535–2543
Weidner C, de Groot JC, Prasad A, Freiwald A, Quedenau C, Kliem M, Witzke A, Kodelja V, Han CT, Giegold S, Baumann M, Klebl B, Siems K, Müller-Kuhrt L, Schürmann A, Schüler R, Pfeiffer AF, Schroeder FC, Büssow K, Sauer S (2012) Amorfrutins are potent antidiabetic dietary natural products. Proc Natl Acad Sci 109:7257–7262
Xie F, Du G, Ma S, Li Y, Wang R, Guo F (2016) Structural elucidation of in vitro metabolites of bavachinin in rat liver microsomes by LC-ESI-MSn and chemical synthesis. Xenobiotica 46:296–306
Acknowledgements
This work was supported by the Excellent Academic Leaders Program of Shanghai (16XD1403500), the Shanghai Science and Technology Committee funding (16401902000) and the program of Shanghai E-Research Institute of Bioactive Constituents in Traditional Chinese Medicine.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
These authors contributed equally: Jingyu Yi, Guoxin Du.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yi, J., Du, G., Zhao, Y. et al. Bavachinin analogues as agonists of pan-peroxisome proliferator-activated receptors. Med Chem Res 27, 1851–1862 (2018). https://doi.org/10.1007/s00044-018-2197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2197-6