Abstract
The utility of 4-isothiocyanato-N-(1-phenyl-1H-pyrazol-5-yl)benzene sulfonamide 2 in the synthesis of some novel thiosemicarbazide, carbamothioate,1,3,4-thiadiazole, azomethine, thiourea, bisthiourea and imidazole derivatives is reported. The structure of the newly synthesized compounds was confirmed on the basis of analytical and spectral data. Some of the prepared compounds were evaluated for their in vitro anticancer activity against Ehrlich ascites carcinoma cells (EAC). It was found that the corresponding 2-acetyl-N-(4-(N-(1-phenyl-1H-pyrazol-5-yl) sulfamoyl)phenyl) hydrazinecarbothioamide 7 with IC50 value (2.14 µg/ml) showed better activity than doxorubicin with IC50 value (43.6 µg/ml) as reference drug.
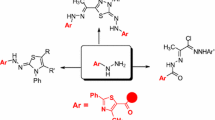
Graphical Abstract
New thiosemicarbazide,carbamothioate,1,3,4-thiadiazole, azomethine, thiourea, bisthiourea and imidazole derivatives containing pyrazole moiety have been synthesized and evaluated for their anticancer activity.







Similar content being viewed by others
References
Abd El-Gawad SM, El-Gaby MSA, Heiba HI, Aly HM, Ghorab MM (2005) Synthesis and radiation stability of some new biologically active hydroquinoline and pyrimido[4,5-b]quinoline derivatives. J Chin Chem Soc 52(6):1227–1236
Abdel-Aziz M, Abuo-Rahma GA, Hassan AA (2009) Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur J Med Chem 44(9):3480–3487. https://doi.org/10.1016/j.ejmech.2009.01.032
Alam MM, Marella A, Akhtar M, Husain A, Yar SMSM, Tanwar O, Saha R, Khanna S, Shaf S (2013) Microwave assisted one pot synthesis of some pyrazole derivatives as a safer anti-inflammatory and analgesic agents. Acta Pol Pharm 70(3):435–441
Anuta V, Nitulescu GM, Dinu-Pîrvu CE, Olaru OT (2014) Biopharmaceutical profiling of new antitumor pyrazole derivatives. Molecules 19:16381–16401. https://doi.org/10.3390/molecules191016381
Brackett CC, Singh H, Block JH (2004) Likelihood and mechanisms of cross-allergenicity between sulfonamide antibiotics and other drugs containing a sulfonamide functional group. Pharmacotherapy 24(7):856–870
Brusick DJ (1984) Ehrlich ascites carcinoma (EAC) cells were maintained in female cytogenetic assays, aberrations and SCE techniques in carcinogenesis and mutagenesis testing. Human Press, Clifton, New Jersey, p 265–276
Castagnolo D, De Logu A, Radi M, Bechi B, Manetti F, Magnani M et al. (2008) Synthesis, biological evaluation and SAR study of novel pyrazole analogs as inhibitors of Mycobacterium tuberculosis. Bioorg Med Chem 16(18):8587–8591
Dias LRS, Salvador RRS (2012) Pyrazole carbohydrazide derivatives of pharmaceutical interest. Pharmaceuticals 5:317–324. https://doi.org/10.3390/ph5030317
El-Gaby MSA, Abd El-Gawad SM, Ghorab MM, Heiba HI, Ali HM (2006) Synthesis and biological activity of some novel thieno[2,3-b] quinoline,quinolino[3’,2’:4,5]thieno[3,2-d]pyrimidine and pyrido[2’,3”:4,5]thieno[2,3-b]quinoline derivatives. Phosphorus Sulfur Silicon 181(2):279–297
El-Gaby MS, Hussien AM, Abu-Shanab FAM, Abdel Rahman MAM (2003) Preparation of some hitherto unknown thiosemicarbazide, thiourea, bisthiourea, benzoazole derivatives bearing quinoxalin-2-yl moiety and evaluate their biological activity. Afinidad 60(506):358–368
El-Gaby MSA, Ismail ZA, Abdel Gawad SM, Aly HM, Ghorab MM (2009) Synthesis of thiazolidinone and thiophene derivatives for evaluation as anticancer. Phosphorus Sulfur Silicon 184:2636–2644
El-Gaby MSA, Micky JA, Taha NM, EL-Sharief MAMSh (2002) Antimicrobial activity of some novel thiourea, hydrazine, fused pyrimidine and 2-(4-substituted) anilino benzoazole derivatives containing sulfonamido moieties. J Chin Chem Soc 49(3):407–414
El-Sabbagh OI, Baraka MM, Ibrahim SM, Pannecouque C, Andrei G, Snoeck R, Balzarini J, Rashad AA (2009) Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur J Med Chem 44(9):3746–3753
Eroglu E (2008) Some QSAR studies for a group of sulfonamide Schiff base as carbonic anhydrase CA II inhibitors. Int J Mol Sci 9:181–197
Faidallah HM, Khan KA, Asiri AM (2011) Synthesis and biological evaluation of new 3-trifluoromethylpyrazolesulfonyl-urea and thiourea derivatives as antidiabetic and antimicrobial agents. J Fluor Chem 132(2):131–137. https://doi.org/10.1016/j.jfluchem.2010.12.009
Gokulan PD, Jayakar B, Alagarsamy V, Raja SV (2012) Synthesis and pharmacological investigation of 5-substituted-3-methylsulfanyl-1H-pyrazole-4-carboxylic acid ethyl esters as new analgesic and anti-inflammatory agents. Arzneimittelforschung 62(10):457–462. https://doi.org/10.1055/s-0032-1321830
Harrison TR (1994) Harrison’s principles of internal medicine, 13th edn. McGraw-Hill, New Delhi, p 604
Ismail ZH, Ghorab MM, Mohamed EMA, Aly HM, El-Gaby MSA (2008) Antitumor activity of some novel 1,2,5-thiadiazole derivatives. Phosphorus Sulfur Silicon 183(10):2541–2554
Kamel MM (2015) Convenient synthesis, characterization, cytotoxicity and toxicity of pyrazole derivatives. Acta Chim Slov 62:136–151. https://doi.org/10.17344/acsi.2014.828
Koca I, Ozgur A, Coskun KA, Tutar Y (2013) Synthesis and anticancer activity of acyl thioureas bearing pyrazole moiety. Bioorg Med Chem 21:3859–3865
Mukerjee AK, Ashare R (1991) Isothiocyanates in the chemistry of heterocycles. Chem Rev 91(1):1–24
Sanjay K, Gyanendra K, Mili K, Avadhesha S, Namita S (2006) Synthesis and evaluation of substituted pyrazoles: potential antimalarials targeting the enoyl-acp reductase of plasmodium falciparum. Synth Commun 36(2):215–226
Sharma S (1989) Isothiocyanates in heterocyclic synthesis. Sulfur Rep 5(5):327–469
Sun J, Zhou Y (2015) Synthesis and antifungal Activity of the derivatives of novel pyrazole carboxamide and isoxazolol pyrazole carboxylate. Molecules 20:4383–4394. https://doi.org/10.3390/molecules20034383
Slatore CG, Tilles SA (2004) Sulfonamide hypersensitivity. Immunol Allergy Clin North Am 24(3):477–490
Tanitame A, Oyamada Y, Ofuji K, Terauchi H, Kawasaki M, Wachi M, Yamagishi J (2005) Synthesis and antibacterial activity of a novel series of DNA gyrase inhibitors: 5-[(E)-2- arylvinyl]pyrazoles. Bioorg Med Chem Lett 15(19):4299–4303
Tilles SA (2001) Practical issues in the management of hypersensitivity reactions: sulfonamides. South Med J 94(8):817–824
Vijesh AM, Isloor AM, Shetty P, Sundershan S, Fun HK (2013) New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur J Med Chem 62:410–415. https://doi.org/10.1016/j.ejmech.2012.12.057
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-VPP- 302
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
El-Gaby, M.S.A., Ghorab, M.M., Ismail, Z.H. et al. Synthesis, structural characterization and anticancer evaluation of pyrazole derivatives. Med Chem Res 27, 72–79 (2018). https://doi.org/10.1007/s00044-017-2035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2035-2