Abstract
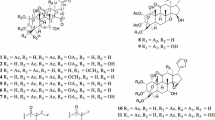
The comparative phytochemical constituents of three Limonium species Limonium myrianthum, Limonium leptophyllum, and Limonium gmelinii afforded a new compound (2R,3S)-2,3,4-trihydroxy-2-methylbutyl gallate (1) and twenty known compounds (2–21). Each species displayed different profiles in their phytochemical constituents. The isolated compounds (1–21) were evaluated for antifungal, antimalarial, and antitrypanosomal activities. Compound 1 showed good activity against chloroquine-resistant and chloroquine-sensitive strains of malaria, while compound 5 displayed moderate antimalarial activity. Compound 14 showed a significant activity against Trypanosoma brucei.



Similar content being viewed by others
References
Akdemir ZŞ, Tatlı İİ, Saracoğlu I, İsmailoğlu UB, Şahin-Erdemli I, Çalış İ (2001) Polyphenolic compounds from Geranium pratense and their free radical scavenging activities. Phytochemistry 56:189–193
Aniya Y, Miyagi C, Nakandakari A, Kamiya S, Imaizumi N, Ichiba T (2002) Free radical scavenging action of the medicinal herb Limonium wrightii from the Okinawa islands. Phytomedicine 9:239–244
Berenbaum MR (1995) The chemistry of defense: theory and practice. Proc Natl Acad Sci USA 92:2–8
Bharate SB, Khan SI, Yunus NA, Chauthe SK, Jacob MR, Tekwani BL, Khan IA, Singh IP (2007) Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg Med Chem 15:87–96
Braca A, Politi M, Sanogo R, Sanou H, Morelli I, Pizza C, De Tommasi N (2003) Chemical composition and antioxidant activity of phenolic compounds from wild and cultivated Sclerocarya birrea (Anacardiaceae) leaves. J Agric Food Chem 51:6689–6695
Bruhn T, Schaumlöffel A, Hemberger Y, Bringmann G (2013) SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 25:243–249
Calzada F, Cerda-García-Rojas CM, Meckes M, Cedillo-Rivera R, Bye R, Mata R (1999) Geranins A and B, new antiprotozoal A-type proanthocyanidins from Geranium niveum. J Nat Prod 62:705–709
Chadwick LR, Nikolic D, Burdette JE, Overk CR, Bolton JL, van Breemen RB, Pauli GF (2004) Estrogens and Congeners from Spent Hops (Humulus lupulus). J Nat Prod 67:2024–2032
Chung SK, Kim YC, Takaya Y, Terashima K, Niwa M (2004) Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. J Agric Food Chem 52:4664–4668
Downie SR, Palmer JD (1994) Phylogenetic relationships using restriction site variation of the chloroplast DNA inverted repeat. In: H-D Behnke, TJ Mabry (eds) Caryophyllales: Evolution and Systematics. Springer-Verlag, Berlin, Heidelberg, p 223–233
Fontana G, Savona G, Rodriguez BN, De La Torre MA (1999) Unusual 6’-fatty acid esters of (24S)-24-ethylcholesta-5, 25-dien-3β-yl β-d-glucopyranoside from Teucrium fruticans. Phytochemistry 50:283–285
Gadetskaya AV, Tarawneh AH, Zhusupova GE, Gemejiyeva NG, Cantrell CL, Cutler SJ, Ross SA (2015) Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia 104:80–85
Gaussian 09, Revision B.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro, Jr. F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, and Fox DJ, Gaussian, Inc., Wallingford CT, 2010
Ghosh SK, Butler MS, Lear MJ (2012) Synthesis of 2-C-methylerythritols and 2-C-methylthreitols via enantiodivergent Sharpless dihydroxylation of trisubstituted olefins. Tetrahedron Lett 53:2706–2708
Guo S, Feng B, Zhu R, Ma J, Wang W (2011) Preparative isolation of three anthraquinones from Rumex japonicus by high-speed counter-current chromatography. Molecules 16:1201–1210
Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P (2004) Comparison of alamar blue and MTT assays for high through-put screening. Toxicol In Vitro 18:703–710
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminf 4:17
Jain SK, Sahu R, Walker LA, Tekwani BL (2012) A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J Visualized Exp 70:e4054
Julkunen-Tiitto R (1989) Phenolic constituents of Salix: a chemotaxonomic survey of further Finnish species. Phytochemistry 28:2115–2125
Kadota S, Takamori Y, Nyein KN, Kikuchi T, Tanaka K, Ekimoto H (1990) Constituents of the leaves of Woodfordia fruticosa Kurz. I. Isolation, structure, and proton and carbon-13 nuclear magnetic resonance signal assignments of woodfruticosin (woodfordin C), an inhibitor of deoxyribonucleic acid topoisomerase II. Chem Pharm Bull 38:2687–2697
Kandil FE, Ahmed KM, Hussieny HA, Soliman AM (2000) A new flavonoid from Limonium axillare. Arch Pharm 333:275–277
Keinänen M, Julkunen-Tiitto R, Rousi M, Tahvanainen J (1999) Taxonomic implications of phenolic variation in leaves of birch (Betula L.) species. Biochem Syst Ecol 27:243–254
Kong NN, Fang ST, Wang JH, Wang ZH, Xia CH (2014) Two new flavonoid glycosides from the halophyte Limonium franchetii. J Asian Nat Prod Res 16:370–375
Korul’kina LM, Shul’ts EE, Zhusupova GE, Abilov ZhA, Erzhanov KB, Chaudri MI (2004) Biologically active compounds from Limonium gmelinii and L. popovii I. Chem Nat Compd 40:465–471
Kozhamkulova ZhA, Radwan MM, Zhusupova GE, Abilov ZhA, Rahadilova SN, Ross SA (2010) Gmelinoside I, a new flavonol glycoside from Limonium gmelinii. Nat Prod Commun 5:1061–1062
Liebezeit G, Künnemann TD, Gad G (1999) Biotechnological potential of North Sea salt marsh plants – a review of traditional knowledge. J Biotechnol 70:77–84
Lin LC, Chou CJ (2000) Flavonoids and phenolics from Limonium sinense. Planta Med 66:382–383
Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA (2004) Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob Agents Chemother 48:4450–4452
Macro Model 9.9, Schrodinger LLC, 2012. (http://www.schrodinger.com/productpage/14/11/) Accessed 2 Feb 2016
Manda S, Khan SI, Jain SK, Mohammed S, Tekwani BL, Khan IA, Vishwakarma RA, Bharate SB (2014) Synthesis, antileishmanial and antitrypanosomal activities of N-substituted tetrahydro-β-carbolines. Bioorg Med Chem Lett 24:3247–3250
Medini F, Fellah H, Ksouri R, Abdelly C (2014) Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. Journal of Taibah University for Science 8:216–224
Miguel MG, Faleiro ML, Guerreiro AC, Antunes MD (2014) Arbutus unedo L.: Chemical and biological properties. Molecules 19:15799–15823
Murray AP, Rodriguez S, Frontera MA, Tomas MA, Mulet MC (2004) Antioxidant metabolites from Limonium brasiliense (Boiss.) Kuntze. Zeitschrift für Naturforschung 59:477–480
Nazir N, Koul S, Qurishi MA, Najar MH, Zargar MI (2011) Evaluation of antioxidant and antimicrobial activities of Bergenin and its derivatives obtained by chemoenzymatic synthesis. Eur J Med Chem 46:2415–2420
Pawlowska AM, De Leo M, Braca A (2006) Phenolics of Arbutus unedo L.(Ericaceae) fruits: identification of anthocyanins and gallic acid derivatives. J Agric Food Chem 54:10234–10238
Rastogi S, Pandey MM, Rawat AKS (2016) Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine 23:1082–1089
Scharbert S, Holzmann N, Hofmann T (2004) Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J Agric Food Chem 52:3498–3508
Seibel J, Moraru R, Götze S, Buchholz K, Na’amnieh S, Pawlowski A, Hecht HJ (2006) Synthesis of sucrose analogues and the mechanism of action of Bacillus subtilis fructosyltransferase (levansucrase). Carbohydr Res 341:2335–2349
Shi S, Zhao Y, Zhou H, Zhang Y, Jiang X, Huang K (2008) Identification of antioxidants from Taraxacum mongolicum by high-performance liquid chromatography–diode array detection–radical-scavenging detection–electrospray ionization mass spectrometry and nuclear magnetic resonance experiments. J Chromatogr A 1209:145–152
Tang XH, Yu F, Liu J, Gao J, Yan LF, Dong MM (2014) Isolation and identification of anti-tumor polysaccharide LSP21 from Limonium sinense (Girard) Kuntze. Int J Biol Macromol 70:138–142
Wink M (2003) Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3–19
Yang LL, Chang CC, Chen LG, Wang CC (2003) Antitumor principle constituents of Myrica rubra var. acuminata. J Agric Food Chem 51:2974–2979
Yang LL, Yen KY, Kiso Y, Hikino H (1987) Antihepatotoxic actions of Formosan plant drugs. J Ethnopharmacol 19:103–110
Yokozawa T, Park CH, Noh JS, Tanaka T, Cho EJ (2009) Novel action of 7-O-galloyl-D-sedoheptulose isolated from Corni Fructus as a hypertriglyceridaemic agent. J Pharm Pharmacol 61:653–661
Yuh-Chi K, Lie-Chwen L, Wei-Jern T, Cheng-Jen C, Szu-Hao K, Yen-Hui H (2002) Samarangenin B from Limonium sinense suppresses herpes simplex virus type 1 replication in vero cells by regulation of viral macromolecular synthesis. Antimicrob Agents Chemother 46:2854–2864
Zhang X, Cambrai A, Miesch M, Roussi S, Raul F, Aoude-Werner D, Marchioni E (2006) Separation of Δ5-and Δ7-phytosterols by adsorption chromatography and semipreparative reversed phase high-performance liquid chromatography for quantitative analysis of phytosterols in foods. J Agric Food Chem 54:1196–1202
Zhusupova GE (2006) Amino-acid and mineral composition of substances from the aerial part and roots of Limonium gmelinii. Chem Nat Compd 42:123–124
Zhusupova GE (2007) Chemical composition of Limonium Mill genus plants and creation of preparations on its basis. Dissertation, Al-Farabi Kazakh National University
Acknowledgements
We are grateful to the Government of the Republic of Kazakhstan and the National Center for Natural Products Research (NCNPR), University of Mississippi, School of Pharmacy, USA for financial support. Also we are grateful to Dr. Melissa Jacob, Dr. Shabana Khan, and Dr. Babu Tekwani for performing antibacterial, antifungal, antimalarial, and antileishmanial bioassays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gadetskaya, A.V., Mohamed, S.M., Tarawneh, A.H. et al. Phytochemical characterization and biological activity of secondary metabolites from three Limonium species. Med Chem Res 26, 2743–2750 (2017). https://doi.org/10.1007/s00044-017-1973-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1973-z