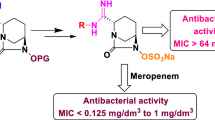
Abstract
Bacterial resistance represents a worldwide emergency threatening the efficacy of all available antibiotics. Among the several resistance mechanisms developed by bacteria, β-lactamase enzymes (BLs), which are able to inactivate most β-lactam core antibiotics, represent a key target to block, thus prolonging antibiotics half-life. Several approaches aimed at inhibiting β-lactamases have been so far undertaken, mainly involving β-lactam-like or covalent inhibitors. Applying a structure-based de novo design approach, we recently discovered a novel, non-covalent and competitive inhibitor of AmpC β-lactamase: lead 1. It has a K i of 1 µM, a ligand efficiency of 0.38 kcal mol−1 and lead-like physical properties. Moreover, it reverts resistance to ceftazidime in bacterial pathogens expressing AmpC and does not up-regulate β-lactamases expression in cell culture. Its features make it a good candidate for chemical optimization: starting from lead 1 crystallographic complex with AmpC, 11 analogs were designed to complement additional AmpC sites, then synthesized and tested against clinically resistant pathogens. While the new inhibitors maintain similar in vitro activity as the starting lead, some of them, in biological assays, extert a higher potency showing improved synergic activity with ceftazidime in resistant clinically isolated strains.
Graphical Abstract



Similar content being viewed by others
References
Babaoglu K, Shoichet BK (2006) Deconstructing fragment-based inhibitor discovery. Nat Chem Biol 2:720–723
Ballatore C, Huryn DM, Smith AB (2013) Carboxylic acid (bio)isosteres in drug design. Chem Med Chem 8:385–395
Bush K, Jacoby A (2010) Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976
Clinical and Laboratory Standards Institute (2010) Performance standards for antimicrobial susceptibility testing. 20th Informational Supplement M100–S209. http://clsi.org/blog/2015/01/08/clsi-publishes-new-antimicrobial-susceptibility-testing-standards/
Cosgrove S, Carmeli Y (2003) The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis 36:1433–1437
Drawz SM, Bonomo RA (2010) Three decades of β-lactamase inhibitors. Clin Microbiol Rev 23:160–201
Eidam O, Romagnoli C, Caselli E, Babaoglu K, Pohlhaus DT, Karpiak J, Bonnet R, Shoichet BK, Prati F (2010) Design, synthesis, crystal structures, and antimicrobial activity of sulfonamide boronic acids as beta-lactamase inhibitors. J Med Chem 53:7852–7863
Eidam O, Romagnoli C, Dalmasso G, Barelier S, Caselli E, Bonnet R, Shoichet BK, Prati F (2012) Fragment-guided design of subnanomolar beta-lactamase inhibitors active in vivo. Proc Natl Acad Sci USA 109:17448–17453
Farina D, Spyrakis F, Venturelli A, Cross S, Tondi D, Costi MP (2014) The inhibition of extended spectrum β-lactamases: hits and leads. Curr Med Chem 21:1405–1434
Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DC, King P, Tsivkovski R, Sun D1, Sabet M, Tarazi Z, Clifton MC, Atkins K, Raymond A, Potts KT, Abendroth J, Boyer SH, Loutit JS, Morgan EE, Durso S, Dudley MN (2015) Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A Serine carbapenemases. J Med Chem 58:3682–3692
Hopkins AL, Groom CR, Alex A (2004) Ligand efficiency: a useful metric for lead selection. Drug Discov Today 9:430–431
Jacoby GA (2009) AmpC beta-lactamases. Clin Microbiol Rev 22:161–182
Kenneth S, Thomson J (2010) Extended-spectrum-β-Lactamase, AmpC, and carbapenemase issues. Clin Microbiol 48:1019–1102
Li G, Zhao G (2006) Allylation of aldehydes and imines: promoted by reuseable polymer-supported sulfonamide of N-glycine. Org Lett 8:633–636
Nukaga M, Kumar S, Nukaga K, Pratt RF, Knox JR (2004) Hydrolysis of third-generation cephalosporins by class C beta-lactamases: structures of a transition state analog of cefotaxime in wild-type and extended spectrum enzymes. J Biol Chem 279:9344–9352
Powers RA, Blazquez J, Weston GS, Morosini MI, Baquero F, Shoichet BK (1999) The complexed structure and antimicrobial activity of a non-beta-lactam inhibitor of AmpC beta-lactamase. Protein Sci 8:2330–2337
Powers RA, Morandi F, Shoichet BK (2002) Structure-based discovery of a novel, noncovalent inhibitor of ampc beta-lactamase. Structure 10:1013–1023
Rice LB, Bonomo RA (2000) Beta-lactamases: which ones are clinically important? Drug Resist Updates 3:178–189
Sassatelli M, Debiton É, Aboab B, Prudhomme M, Moreau P (2006) Synthesis and antiproliferative activities of indolin-2-one derivatives bearing amino acid moieties. Eur J Med Chem 41:709–716
Tondi D, Calò S, Shoichet BK, Costi MP (2010) Structural study of phenyl boronic acid derivatives as AmpC β-lactamase inhibitors. Bioorg Med Chem Lett 20(11):3416–3419
Tondi D, Cross S, Venturelli A, Costi MP, Cruciani G, Spyrakis F (2016) Decoding the structural basis for carbapenem hydrolysis by class A β- lactamases: fishing for a pharmacophore. Curr Drug Targets 17:983–1005
Tondi D, Morandi F, Bonnet R, Costi MP, Shoichet BK (2005) Structure-based optimization of a Non-β-lactam lead results in inhibitors that do not up-regulate β-lactamase expression in cell culture. J Am Chem Soc 127:4632–4639
Tondi D, Powers RA, Caselli E, Negri MC, Blázquez J, Costi MP, Shoichet BK (2001) Structure-based design and in-parallel synthesis of inhibitors of AmpC beta-lactamase. Chem Biol 8:593–611
Tondi D, Venturelli A, Bonnet R, Pozzi C, Shoichet BK, Costi MP (2014) Targeting class A and C Serine β-lactamases with a broad-spectrum boronic acid derivative. J Med Chem 57:5449–5458
Venturelli A, Tondi D, Cancian L, Morandi F, Cannazza G, Segatore B, Prati F, Amicosante G, Shoichet BK, Costi MP (2007) Optimizing cell permeation of an antibiotic resistance inhibitor for improved efficacy. J Med Chem 50:5644–5654
World Health Organization (2014) Antimicrobial resistance: global report on surveillance. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf. Accessed 23 Feb 2016
Acknowledgements
This work was supported by NIH GM63815, by FAR 2014, University of Modena to DT, by Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, the Spanish Network for Research in Infectious Diseases RD12/0015/0029 and Fondo de Investigación Sanitaria Grant PI13/00063. C.I-Q. was supported by a grant from “Becas para estudios de doctorado en el extranjero” from Chile Scholarship Program (72110933), National Council for Scientific and Technological Research (CONICYT), Chilean Government. The authors acknowledge the “Fondazione Cassa di Risparmio di Modena” for funding the HPLC-ESI-QTOF system at the Centro Interdipartimentale Grandi Strumenti of the University of Modena and Reggio Emilia. We acknowledge Dr. Pasquale Linciano for support in NMR compounds characterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Genovese, F., Lazzari, S., Venturi, E. et al. Design, synthesis and biological evaluation of non-covalent AmpC β-lactamases inhibitors. Med Chem Res 26, 975–986 (2017). https://doi.org/10.1007/s00044-017-1809-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1809-x