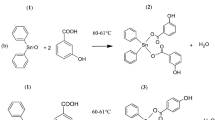
Abstract
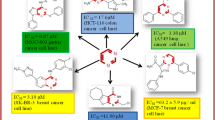
Several halogenated 2-amino-4H-benzo[h]chromene derivatives were synthesized and evaluated their cytotoxicity. The structures of the synthesized compounds were established on the basis of spectral data. The in vitro antitumor activity of the synthesized compounds against the cell lines MCF-7, HCT-116, and HepG-2 was investigated in comparison with the reference drugs vinblastine, colchicine, and doxorubicin using microculture tetrazolium colorimetric assay. It was found that some halogenated 4H-benzo[h]chromene derivatives showed the highest antitumor activity as compared with the reference drugs. The structure–activity relationship studies reported that the substitution at 4-position in the 4H-benzo[h]chromene nucleus with the specific halogen groups and lipophilicity increases the ability of the molecule against the different cell lines.





Similar content being viewed by others
References
Avendano C, Menendez JC (2008) Medicinal Chemistry of Anticancer Drugs, 1st edn., p. 1 (Chapter 1), Elsevier Publishers, ISBN: 978-0-444-52824-7
Abd-El-Aziz AS, El-Agrody AM, Bedair AH, Corkery TC, Ata A (2004) Synthesis of hydroxyquinoline derivatives, aminohydroxychromene, aminocoumarin and their antimicrobial activities. Heterocycles 63:1793–1812
Al-Ghamdi AM, Abd EL-Wahab AHF, Mohamed HM, El-Agrody AM (2012) Synthesis and antitumor activities of 4H-pyrano[3,2-h]quinoline-3-carbonitrile, 7H-pyrimido[4′,5′:6,5]-pyrano[3,2-h]quinoline, and 14H-pyrimido[4′,5′:6,5]pyrano[3,2-h][1,2,4]triazolo[1,5-c]quinoline derivatives. Lett Drug Des Discov 9:459–470
El-Agrody AM (1994) Condensation reactions of a-cyanocinnamonitriles with naphthols: synthesis of naphthopyranopyrimidines and a naphthopyranone. J Chem Res (S) 280–281
El-Agrody AM, Abd El-Mawgoud HK, Fouda AM, Khattab ESAEH (2016b) Synthesis, in-vitro cytotoxicity of 4H-benzo[h]chromene derivatives and structure–activity relationships of 4-aryl group and 3-, 7-positions. Chem Pap 70:1279–1292
El-Agrody AM, Abd-Rabboh HSM, Al-Ghamdi AM (2013a) Synthesis, antitumoractivity, and structure–activity relationship of some 4H-pyrano[3,2-h]quinoline and 7H-pyrimido-[4’,5’:6,5]pyrano[3,2-h]quinoline derivatives. Med Chem Res 22:1339–1355
El-Agrody AM, Fouda AM, Al-Dies AM (2014a) Studies on the synthesis, in vitro antitumor activity of 4H-benzochromene, 7Hbenzo[h]chromeno[2,3-d]pyrimidine derivatives and structure activity relationships of the 2-,3- and 2,3-positions. Med Chem Res 23:3187–3199
El-Agrody AM, Fouda AM, Khattab ESAEH (2013b) Synthesis, antitumor activity of 2-amino-4H-benzo[h]chromene derivatives and structure–activity relationships of the 3- and 4-positions. Med Chem Res 22:6105–6120
El-Agrody AM, Halawa AH, Fouda AM, Al-Dies, AM (2016a) Anti-proliferative activity of novel 4H-benzo[h]chromenes, 7H-benzo[h]chromeno[2,3-d]pyrimidines and the structure–activity relationships of the 2-, 3-positions and fused rings at the 2, 3-positions. J Saudi Chem Soc. doi:10.1016/j.jscs.2016.03.002
El-Agrody AM, Khattab ESAEH, Fouda AM, Al-Ghamdi AM (2012) Synthesis and antitumor activities of certain novel 2-amino-9-(4-halostyryl)-4H-pyrano[3,2-h]quinoline derivatives. Med Chem Res 21:4200–4213
El-Agrody AM, Khattab ESAEH, Fouda AM (2014b) Synthesis, structure–activity relationship (SAR) studies on some 4-Aryl-4H-chromenes and relationship between lipophilicity and antitumor activity. Lett Drug Des Discov 11:1167–1176
El-Agrody AM, Sabry NM, Motlaq SS (2011) Synthesis of some new 2-substituted 12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine, 3-ethoxycarbonyl-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-2-one, ethyl 2-formylamino/acetylamino-4H-chromene-3-carboxylate and some of their antimicrobial activities. J Chem Res 35:77–83
Halawa AH, Fouda AM, Al-Dies AM, El-Agrody AM (2016) Synthesis, biological evaluation and molecular docking studies of 4Hbenzo[h]chromenes, 7H-benzo[h]chromeno[2,3-d]pyrimid-ines as antitumor agents. Lett Drug Des Discov 13:77–88. F Deriv
Kheirollahi A, Pordeli M, Safavi M, Mashkouri S, Naimi-Jamal MR, Ardestani SK (2014) Cytotoxic and apoptotic effects of synthetic benzochromene derivatives on human cancer cell lines. Naunyn-Schmiedeberg’s Arch Pharmacol 387:1199–1280
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nepali K, Sharma S, Sharma M, Bedi PMS, Dhar KL (2014) Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem 77:422–487
Panda D, Singh JP, Wilson L (1997) Suppression of microtubule dynamics by LY290181 a potential mechanism for its antiproliferative action. J Biol Chem 272:7681–7687
Rahman AU, Choudhary MI, Thomsen WJ (2001) Bioassay technique for drug development. Harwood Academic Publishers ISBN 0-203-34349-2 (Adobe e-Reader Format), ISBN 90- 5823-051-1 (Print Edition)
Sabry NM, Mohamed HM, Khattab ESAEH, Motlaq SS, El-Agrody AM (2011) Synthesis of 4H-chromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur J Med Chem 46:765–772
Snedecor GM, Cochran WG (1982) Statistical methods, 7th edn. Lowa state University Press, Ames, pp 325–330
Wood DL, Panda D, Wiernicki TR, Wilson L, Jordan MA, Singh JP (1997) Inhibition of mitosis and microtubule function through direct tubulin binding by a novel antiproliferative naphthopyran LY290181. Mol Pharmacol 52:437–444
Acknowledgments
This research was supported by a program to support research and researchers at King Khalid University, Abha, Saudi Arabia and No. (KKU-SCI-11-028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
El-Agrody, A.M., Fouda, A.M. & Khattab, E.S.A.E.H. Halogenated 2-amino-4H-benzo[h]chromene derivatives as antitumor agents and the relationship between lipophilicity and antitumor activity. Med Chem Res 26, 691–700 (2017). https://doi.org/10.1007/s00044-016-1773-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1773-x