Abstract
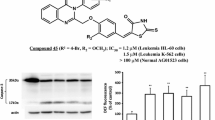
Novel N-substituted rhodanines 2a–g were synthesized by conventional and microwave-assisted methods and tested for their anticancer activity. Structure–activity relationship of the synthesized rhodanine 2a–g as antiproliferative agents was investigated. The results revealed that all the seven compounds showed potent antiproliferative activity in a concentration-dependent manner on leukemic cell line K562. Among the tested compounds, 2b was found to be more potent when compared by trypan blue and MTT assay. IC50 values of 2b using trypan blue and MTT assay were found to be 11.1 and 20.3 µg/ml, respectively. A dose-dependent increase in the LDH release was also observed upon treatment with 2a–g. Cell cycle analysis revealed that 2b affects DNA replication and leads to accumulation of cells in G 0 and decline of G 2/M, G 1 and S phases which indicates apoptosis. The selective cytotoxic activity against human chronic myelogenous cell line (K562), via apoptosis, suggests that compound 2b is a promising scaffold for the development of novel anticancer drug.






Similar content being viewed by others
References
Alizadeh A, Zohreh N (2009) A novel multicomponent method for the synthesis of 2-thioxo-1,3-thiazolidin-4-ones. Synlett 13:2146–2148
Alizadeh A, Rostamnia S, Zohreh N, Hosseinpour R (2009) A simple and effective approach to the synthesis of rhodanine derivatives via three-component reactions in water. Tetrahedron Lett 50:1533–1535
Anumala UR, Gu J, Monte FL, Kramer T, Haußen RB, Hölzer J, Goetschy-Meyer V, Schön C, Mall G, Hilger I, Czech C, Herms J, Schmidt B (2013) Fluorescent rhodanine-3-acetic acids visualize neurofibrillary tangles in Alzheimer’s disease brains. Bioorg Med Chem 21:5139–5144
Arung ET, Wicaksono BD, Handoko YA, Kusuma IW, Yulia D, Ferrry S (2009) Anti- cancer properties of diethylether extract of wood from sukun (artocarpus altilis) in human breast cancer (T47D) cells). J Immunol Methods 8:217–324
Bernardo PH, Xu J, Wan KF, Sivaraman T, Krishnamurthy J, Mok HYK, Yu VC, Chai CLL (2009) Rhodanine-based Pan-Bcl-2 inhibitors and Mcl-1-specific inhibitors as anti-cancer compounds (WO 2010024783). International Patent no (PCT/SG2009/000301)
Bernardo PH, Sivaraman T, Wan K, Xu J, Krishnamurthy J, Song CM, Liming T, Chin JSF, Lim DSW, Mok HYK, Yu VC, Tong JC, Chai CLL (2011) Synthesis of a rhodanine-based compound library targeting Bcl-XL and Mcl-1. Pure Appl Chem 83:723–731
Brooker IAS, Keyes GHF, Roch N (1950) Acid merocyanine dyes. US Patented (US2493747 A). 605:472
Brown FC, Bradsher CK (1951) Mildew-preventing activity of rhodanine derivatives. Nature 168:171–172
Brown FC, Bradsher CK, Morgan EC, Tetenbaum M, WilderJr P (1956) Some 3-substituted rhodanines. J Am Chem Soc 78:384–388
David SG (2009) Method using lifespan-altering compounds for altering the lifespan of eukaryotic organisms, and screening for such compounds (US 20090163545 A1 20090625). US Patent App Pub USA
Frankov IA, Kirillov MV, Sokolova TN, Skupskaya RV, Kharitonovich AN, Chizhevskaya II (1985) Synthesis and pharmacoloical properties of 3-carboxyalkylrhodanines containing alkylating moieties. Pharm Chem J 19:544–547
Guo M, Zheng CJ, Song MX, Wu Y, Sun LP, Li YJ, Liu Y, Piao HR (2013) Synthesis and biological evaluation of rhodanine derivatives bearing a quinoline moiety as potent antimicrobial agents. Bioorg Med Chem Lett 23:4358–4361
Jacobine AM, Posner GH (2011) Three-component, one-flask synthesis of rhodanine (thiazolidinone) derivatives. J Org Chem 76:8121–8125
Kamila S, Ankati H, Harry E, Edward RB (2012) A facile synthesis of novel 3-(aryl/alkyl-2-ylmethyl)-2-thioxothiazolidin-4-ones using microwave heating. Tetrahedron Lett 53:2195–2198
Kaminsky D, Bednarczyk-Cwynar B, Vasylenko O, Kazakova O, Zimenkovsky B, Zaprutko L, Lesyk R (2012) Synthesis of new potential anticancer agents based on 4-thiazolidinone and oleanane scaffolds. Med Chem Res 21:3568–3580
Kavya R, Vladimir NY, Alexandra SN, Igor VZ, Mikhail MK, Leonid VK, Adrian E, Srinivas O, Nouri N (2010a) Design, synthesis and structure-activity studies of rhodanine derivatives as HIV-1 integrase inhibitors. Molecules 15:3958–3992
Kavya R, Vladimir NY, Alexandra SN, Igor VZ, Mikhail MK, Leonid VK, Adrian E, Srinivas O, Nouri N (2010b) Design, synthesis and structure-activity studies of rhodanine derivatives as HIV-1 integrase inhibitors. Molecules 15:3958–3992
Korzeniewski C, Callewaert DM (1983) An enzyme-release assay for natural cytotoxicity. J Immunol Methods 64:313–320
Lesyk RB, Zimenkovsky BS (2004) 4-Thiazolidones: centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr Org Chem 8:1547–1577
Martinez GA, Dorronsoro DI, Alonso CM, Panizo DPG, Fuertes HA, Perez PMJ, Medina PM (2003) Preparation of thiadiazolidines and related compounds as GSK-3 inhibitors (US 20030195238 A1 20031016). U.S. Patent App Pub USA
Moorthy BT, Ravi S, Srivastava M, Chiruvella KK, Hemlal H, Joy O, Raghavan SC (2010) Novel rhodanine derivatives induce growth inhibition followed by apoptosis. Bioorg Med Chem Lett 20:6297–6301
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nitsche C, Klein CD (2012) Aqueous microwave-assisted one-pot synthesis of N-substituted rhodanines. Tetrahedron Lett 53:5197–5201
Prashantha Kumar BR, Baig NR, Sudhir S, Kara K, Karana M, Pankaj M, Joghee NM (2012) Discovery of novel glitazones incorporated with phenylalanine and tyrosine: synthesis, antidiabetic activity and structure-activity relationships. Bioorg Med Chem 45:12–28
Ravi S, Chiruvella KK, Rajesh K, Prabhu V, Raghavan SC (2010) 5-Isopropylidene-3-ethyl rhodanine induce growth inhibition followed by apoptosis in leukemia cells. Eur J Med Chem 45:2748–2752
Shahabuddin MS, Nambiar M, Choudhary B, Advirao GM, Raghavan SC (2009) A novel DNA intercalator, butylamino-pyrimido [4′,5′: 4,5] selenolo (2,3-b) quinoline, induces cell cycle arrest and apoptosis in leukemic cells. Invest New Drugs 28:35–48
Vivek P, Rajender SV (2008) Aqueous microwave chemistry: a clean and green synthetic tool for rapid drug discovery. Chem Soc Rev 37:1546–1557
Volynets GP, Bdzhola VG, Golub AG, Synyugin AR, Chekanov MA, Kukharenko OP, Yarmoluk SM (2013) Rational design of apoptosis signal regulating kinase 1 inhibitors: discovering novel structural scaffold. Eur J Med Chem 61:104–115
Wang S, Zhao Y, Zhu W, Liu Y, Goo K, Gong P (2012) Synthesis and anticancer activity of indolin-2-one derivatives bearing the 4-thiazolidinone moiety. Arch Pharm Chem Life Sci 345:73–80
Xing C, Wang L, Tang X, Sham YY (2007) Development of selective inhibitors for anti-apoptotic Bcl-2 proteins from BHI-1. Bioorg Med Chem 15:2167–2176
Acknowledgments
The authors thank Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India, for funding the project (No. SR/S1/OC-28/2011). The authors thank SAIF-STIC, Cochin, for NMR facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali Muhammad, S., Ravi, S. & Thangamani, A. Synthesis and evaluation of some novel N-substituted rhodanines for their anticancer activity. Med Chem Res 25, 994–1004 (2016). https://doi.org/10.1007/s00044-016-1545-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1545-7