Abstract
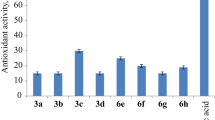
A new class of chalcones, aurones and flavones derived from carbazole is designed as potential antimicrobial and antioxidant agents. Synthesis of (Z)-2-((9-ethyl-9H-carbazol-3-yl)methylene)benzofuran-3(2H)-ones and 2-(9-ethyl-9H-carbazol-3-yl)-4H-chromen-4-ones was carried out by the oxidation of (E)-3-(9-ethyl-9H-carbazol-3-yl)-1-(2-hydroxyphenyl)prop-2-en-1-ones under microwave irradiation and conventional heating. All the newly synthesized compounds were characterized on the basis of IR, 1H NMR, 13C NMR, mass and analytical data. All the synthesized compounds were evaluated for their antibacterial, antifungal and antioxidant activities. Synthesized compounds were screened in vitro for antibacterial activity against two gram-positive bacterial strains like Staphylococcus aureus and Bacillus subtilis and two gram-negative bacterial strains like Escherichia coli and Klebsiella pneumonia and antifungal activity by inhibitory action against three fungal strains like Fusarium oxysporum, Aspergillus niger and Aspergillus flavus. The synthesized compounds were also evaluated for their DPPH radical scavenging activity. All the newly synthesized compounds have shown good antibacterial, antifungal and antioxidant activities.







Similar content being viewed by others
References
Ashok D, Ravi S, Sreenivas P (2013) Solvent-free microwave-assisted synthesis of 3-((E)-3-(2,4-dihydroxy-5-((E)-3-(4-oxo-4H-3-chromenyl)-2-propenoyl)phenyl)-3-oxo-1-propenyl)-4H-chromen-4-ones. Indian J Heterocycl Chem 23:11–14
Ashok D, Gandhi DM, Srinivas G, Vikas KA (2014) Microwave-assisted synthesis of novel 1,2,3-triazole derivatives and their antimicrobial activity. Med Chem Res 23:3005–3018
Bano S, Javed K, Ahmad S, Rathish IG, Singh S, Chaitanya M, Arunasree KM, Alam MS (2013) Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur J Med Chem 65:51–59
Barbosa TP, Suervy SCO, Amorim FM, Rodrigues YKS, De Assis PAC, Caldas JPA, Oliveira MR, Vasconcellos MLAA (2011) Design, synthesis and antileishmanial in vitro activity of new series of chalcones-like compounds: a molecular hybridization approach. Bioorg Med Chem 19(14):4250–4256
Boumendjel A, Boccard J, Carrupt PA, Nicolle E, Blanc M, Geze A, Choisnard L, Wouessidjewe D, Matera EL, Dumontet C (2008) Antimitotic and antiproliferative activities of chalcones: forward structure–activity relationship. J Med Chem 51:2307–2310
Cheng H, Zhang L, Liu Y, Chen S, Cheng H, Lu X, Zheng Z, Zhou GC (2010) Design, synthesis and discovery of 5-hydroxyaurone derivatives as growth inhibitors against HUVEC and some cancer cell lines. Eur J Med Chem 45:5950–5957
Cotelle N, Bemier JL, Catteau JP, Pommery J, Wallet JC, Gaydou EM (1996) Antioxidant properties of hydroxy flavones. Free Radic Biol Med 20:35–43
Detsi A, Majdalani M, Kontogiorgis CA, Hadjipavlou-Litina D, Kefalas P (2009) Natural and synthetic 2'-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg Med Chem 17(23):8073–8085
Groot HD, Rauen U (1998) Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol 12:249–255
Hadj-esfandiari N, Navidpour L, Shadnia H, Amini M, Samadi N, Faramarzid MA, Shafiee A (2007) Synthesis, antibacterial activity and quantitative structure–activity relationships of new (Z)-2-(nitroimidazolylmethylene)-3(2H)-benzofuranone derivatives. Bioorg Med Chem 17:6354–6363
Itoigawa M, Kashiwada Y, Ito C, Furukawa H, Tachibana Y, Bastow KF, Lee KH (2000) Antitumor agents. 203. Carbazole alkaloid murrayaquinone A and related synthetic carbazolequinones as cytotoxic agents. J Nat Prod 63:893–897
Kayser O, Kiderlen AF (1998) Leishmanicidal activity of aurones. Tokai J Exp Clin Med 23:423–426
Li N, Liu JH, Zhang J, Yu BY (2008) Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J Agric Food Chem 56:1429–1433
Lim H, Jin JH, Park H, Kim HP (2011) New synthetic anti-inflammatory chrysin analog, 5,7-dihydroxy-8-(pyridine-4yl)flavones. Eur J Pharmacol 670:617–622
Lin HJ, De YH, Fang L, Wei X, Xia Z (2008) Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L) Meeb. Bioorg Med Chem 16:1337–1344
Morimoto M, Fukumoto H, Nozoe T, Hagiwara A, Komai K (2007) Synthesis and insect antifeedant activity of aurones against Spodoptera litura larvae. J Agric Food Chem 55:700–704
Okombi S, Rival D, Bonnet S, Mariotte AM, Perrier E, Boumendjel A (2006) Discovery of benzylidenebenzofuran-3(2H)-one (aurones) as inhibitors of tyrosinase derived from human melanocytes. J Med Chem 49:329–333
Radha K, Youra K, Chul HK, Kyungrook K, Jung AK, Eung SL (2012) Hydroxychalcones as potential anti-angiogenic agent. Bull Korean Chem Soc 33:2925–2929
Shenvi S, Kumar K, Hatti KS, Rijesh K, Diwakar L, Reddy GC (2013) Synthesis, anticancer and antioxidant activities of 2,4,5-trimethoxy chalcones and analogues from asaronaldehyde: structure activity relationship. Eur J Med Chem 62:435–442
Shin SY, Shin JS, Lee YS (2011) Synthesis of aurones and their inhibitory effects on nitric oxide and PGE productions in LPS-induced RAW 264.7 cells. Bioorg Med Chem Lett 21:4520–4523
Thomas MG, Lawson C, Allanson NM, Leslie BW, Bottomley JR, McBride A, Olusanya OA (2003) A series of 2(Z)-2-benzylidene-6,7-dihydroxybenzofuran-3[2H]-ones as inhibitors of chorismate synthase. Bioorg Med Chem Lett 3:423–426
Tomohiro I, Kenji O, Munekazu I, Yoshinori N, Yukihiro A (2008) Inhibitory effects of polymethoxy flavones isolated from Citrus reticulate on degranulation in rat basophilic leukemia RBL-2H3: enhanced inhibition by their combination. Bioorg Med Chem 16:7592–7598
Tran TD, Do TH, Tran NC, Ngo TD, Huynh TNP, Tran CD, Thai KM (2012) Synthesis and anti Methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorg Med Chem Lett 22:4555–4560
Wu J, Li J, Cai Y, Pan Y, Ye F, Zhang Y, Zhao Y, Yang S, Li X, Lian G (2011) Evaluation and discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J Med Chem 54(23):8110–8123
Xuea B, Lib J, Chaic Q, Liuc Z, Chenb L (2008) Effect of total flavonoid fraction of Astragalus complanatus R. Brown on angiotensin II-induced portal-vein contraction in hypertensive rats. Phytomedicine 15:759–762
Acknowledgments
We are thankful to the Head, Department of Chemistry, Osmania University, for providing laboratory facilities and CFRD OU, for providing spectral analysis. S.R. is thankful to the Council of Scientific and Industrial Research, New Delhi, for the award of senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashok, D., Ravi, S., Ganesh, A. et al. Microwave-assisted synthesis and biological evaluation of carbazole-based chalcones, aurones and flavones. Med Chem Res 25, 909–922 (2016). https://doi.org/10.1007/s00044-016-1537-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1537-7