Abstract
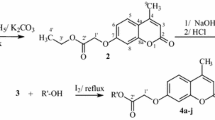
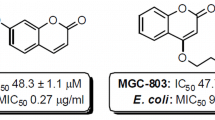
Oxadiazoles are heterocyclic compounds with a variety of application in many pharmaceuticals and agrochemical products. We report herein the convenient synthesis of 4-[(3-aryl-1,2,4-oxadiazol-5-yl)methoxy]-coumarins (4a–f), 6-[(3-aryl-1,2,4-oxadiazol-5-yl)methoxy]-4-methylcoumarins (7a–f) and 7-[(3-aryl-1,2,4-oxadiazol-5-yl)methoxy]-4-phenylcoumarins (10a–f) in high yields by one-pot condensation reaction of esters with amidoximes. The structures of the synthesized compounds were established on the basis of IR, NMR and mass spectrometry. The antibacterial and antifungal activities of synthesized compounds were evaluated.
Graphical Abstract




Similar content being viewed by others
References
Atwal KS, Grover JG, Ahmed ZS, Ferrara NF, Harper WT, Sleph GP, Dzwonczyk S, Russell DA, Moreland S, Mecullough RJ, Normandin ED (1993) Cardioselective anti-ischemic ATP-sensitive potassium channel openers. J Med Chem 36:3971
Ayer WA, Nozawa K (1990) Taxonomy and chemistry of a new fungus from bark beetle infested Pinus contorta var. latifolia. Part 2. Arthrographol, the metabolite inhibitory to ophiostoma clavigerum. Can J Microbiol 36:83
Barahman M, Farzaneh T (2014) Mild and efficient one pot synthesis of 3,5-disubstituted 1,2,4-oxadiazoles from nitriles mediated by K3PO4. Synth Commun 44:188–194
Borg S, Vollinga RC, Laborre M, Payza K, Terenius L, Luthman K (1999) Design, synthesis, and evaluation of Phe-Gly mimetics: heterocyclic building blocks for pseudopeptides. J Med Chem 42:4331–4342
Borges F, Roleira F, Milhazes N, Santana L, Uriarte E (2005) Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr Med Chem 12(8):887–916
Chimichi S, Boccalini M, Cosimelli B (2002) A new convenient route to 2-oxoethoxycoumarins: key intermediates in the synthesis of natural products. Tetrahedron 58(24):4851–4858
Chimirri A, Grasso S, Montforte AM, Rao A, Zappala M (1996) Synthesis and antitumor activity of 1,2,4-oxadiazoline derivatives. Farmaco 51:125–129
Clitherow JW, Beswick P, Irving WJ, Scopes DIC, Barnes JC, Clapham J, Brown JD, Evans DJ, Hayes AG (1996) Novel 1,2,4-oxadiazoles as potent and selective histamine H3 receptor antagonists. Bioorg Med Chem Lett 6:833–838
De Freitas JJR, De Freitas JCR, Da Silva LP, De Freitas FJR, Kimura GYV, Srivastava RM (2007) Microwave-induced one-pot synthesis of 4-[3-(aryl)-1,2,4- oxadiazol-5-yl]-butan-2-ones under solvent free conditions. Tetrahedron Lett 48:6195–6198
Dexeus FH, Logothetis CJ, Sella A, Fitz K, Amato R, Reuben JM, Dozier N (1990) Phase II study of coumarin and cimetidine in patients with metastatic renal cell carcinoma. J Clin Oncol 8:325
Evans MD, Ring J, Schoen A, Bell A, Edwards P, Berthelot D, Nicewonger R, Baldino CM (2003) The accelerated development of an optimized synthesis of 1,2,4-oxadiazoles: application of microwave irradiation and statistical design of experiments. Tetrahedron Lett 44:9337–9341
Finn G, Creaven B, Egan D (2003) Modulation of mitogen-activated protein kinases by 6-nitro-7-hydroxycoumarin mediates apoptosis in renal carcinoma cells. Eur J Pharmacol 481:159
Finn GJ, Creaven BS, Egan DA (2004) Investigation of intracellular signalling events mediating the mechanism of action of 7-hydroxycoumarin and 6-nitro-7-hdroxycoumarin in human renal cells. Cancer Lett 205:69
Gareau Y, Jutaeu H, Mackay B (2004) Preparation of 7-hydroxy-4-phenyl coumarin. Merck Frosst Canada and Co. WO2004/108720
Jochims JC (1996) In: Katritzky AR, Rees CW, Scriven EVF (eds) Comprehensive heterocyclic chemistry II, vol 4, chap 4. Pergamon: London, pp 179–228
Kaboudin B, Malekzadeh L (2011) Organic reactions in water: an efficient method for the synthesis of 1,2,4-oxadiazoles in water. Tetrahedron Lett 52:6424–6426
Katritzky AR, Shestopolov AA, Suzuki K (2005) A convenient synthesis of chiral 1,2,4-oxadiazoles from N-protected (α-aminoacyl)benzotriazoles. Arkivoc 1:36–55
Keating GJ, Kennedy RO (1997) Coumarins: biology, applications and mode of action. John Wiley & Sons, New York, pp 23–66
Kempen I, Papapostolou D, Thierry N, Pochet L, Counerotte S, Masereel B, Foidart JM, Reboud-Ravaux M, Noel N, Pirotte B (2003) 3-Bromophenyl 6-acetoxymethyl-2-oxo-2H-1-benzopyran-3-carboxylate inhibits cancer cell invasion in vitro and tumour growth in vivo. Br J Cancer 88:1111
Kontogiorgis C, Hadjipavlou-Litina D (2003) Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J Enzyme Inhib Med Chem 18:63
Korbonits D, Kanzel-Szvoboda I, Gonczi C, Simon K, Kolonits P (1989) Ring transformation of 1,2-disubstituted 4(1H)-quinazolone oximes to 3,5-disubstituted 1,2,4-oxadiazoles. Chem Ber 122:1107–1112
Kumar D, Patel G, Johnson EO, Shah K (2009) Synthesis and anticancer activities of novel 3,5-disubstituted 1,2,4-oxadiazoles. Bioorg Med Chem Lett 19:2739–2741
Luthman K, Borg S, Hacksell U (1999) Synthesis and use of pseudopeptides derived from 1,2,4-oxadiazole-, 1,3,4-oxadiazole-, and 1,2,4-triazole-based dipeptidomimetics. Methods Mol Med 23:1–23
Maria ER, Albertina M, Ramiro V, Natalia G, Graciela F, Lidia P, Emilio R, Carina S, Carlos D (2008) Structural insights into hydroxycoumarin-induced apoptosis in U-937 cells. Bioorg Med Chem 16:2665–2675
Mathivanan P, Christine GM, Sang PH, Andrew DW (2012) N-Aryl pyrrolidinonyl oxadiazoles as potent mGluR5 positive allosteric modulators. Bioorg Med Chem Lett 22:5658–5662
Maxwell A (1993) The interaction between coumarin drugs and DNA gyrase. Mol Microbiol 9:681
Morita H, Dota T, Kobayashi J (2004) Antimitotic activity of glaupalol-related coumarins from Glaucidium palmatum. Bioorg Med Chem Lett 14:3665
Murray RDH (1989) Coumarins. Nat Prod Rep 6:591–624
Murray RDH, Mendez J, Brown SA (1982) The natural coumarins: occurrence, chemistry and biochemistry. Wiley, Chichester
Negwer M (1987) Organic-chemical drugs and their synonyms. Academie-Verlag, Berlin
Nicolaides DN, Fylaktakidou KC, Litinas KE, Hadjipavlou LD (1998) Synthesis and biological evaluation of several coumarin-4-carboxamidoxime and 3-(coumarin-4-yl)-1,2,4-oxadiazole derivatives. Eur J Med Chem 33:715–724
Orlek BS, Blaney FE, Brown F, Clark MS, Hadley MS, Hatcher J, Riley GJ, Rosenberg HE, Wadsworth HJ, Wyman P (1991) Comparison of azabicyclic esters and oxadiazoles as ligands for the muscarinic receptor. J Med Chem 34:2726–2735
Pei LZ, Le W, Xiao LZ, Xiaoqin H, Chang GZ, Guang FY (2010) Subnanomolar inhibitor of cytochrome bc1 complex designed via optimizing interaction with conformationally flexible residues. J Am Chem Soc 132:185–194
Peng W, Jianzhen L, Hualu X, Guisen Z (2012) Synthesis and anticancer activity of novel 5-(indole-2-yl)-3-substituted 1,2,4-oxadiazoles. Drug Discov Ther 6(3):133–139
Pfefferle W, Anke H, Bross D, Steffan B, Vinaden R, Steglich W (1990) Asperfuran, a novel antifungal metabolite from Aspergillus oryzae. J Antibiot 43:648
Pushpak M, Bekington M (2006) Synthesis of substituted 4-(3-alkyl-1,2,4-oxadiazol-5-ylmethyl)-3,4-dihydro-2H-1,4-benzoxazines and 4-(1H-benzimidazol-2-ylmethyl)-3,4-dihydro-2H-1,4-benzoxazines. Tetrahedron Lett 47:7823–7826
Raju R, Subbu PJ, Carlos M (2011) Antimycobacterial activity of novel 1,2,4-oxadiazole-pyranopyridine/chromene hybrids generated by chemoselective 1,3-dipolar cycloadditions of nitrile oxides. Bioorg Med Chem 19:3444–3450
Swinbourne JF, Hunt H, Klinkert G (1979) Advances in indolizine chemistry. Adv Heterocycl Chem 23:103–170
Tsuge O, Urano S, Oe K (1980) Reactions of trimethylsilyl azide with heterocumulenes. J Org Chem 45:5130–5136
Vu CB, Corpuz EG, Merry TJ, Pradeepan SG, Bartlett C, Bohacek RS, Botfield MC, Eyermann CJ, Iynch BA, MacNeil IA, Ram MK, Van SMR, Violette S, Sawyer TK (1999) Discovery of potent and selective SH2 inhibitors of the tyrosine kinase ZAP-70. J Med Chem 42:4088–4098
Yu D, Suzuki M, Xie L, Morris NSL, Lee KH (2003) Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med Res Rev 23:322
Zabradnik M (1992) The production and application of fluorescent brightening agents. Wiley, New York
Zembower DE, Liao S, Flavin MT, Xu ZQ, Stup TL, Buckheit RW, Khilevich A (1997) Structural analogues of the calanolide anti-HIV agents. Modification of the trans-10,11-dimethyldihydropyran-12-ol ring (ring C). J Med Chem 1997:40
Zhang HE, Kasibhatla S, Kuemmerle J, Kemnitzer W, Ollis MK, Qiu L, Crogan GC, Tseng B, Drewe J, Cai SX (2005) Discovery and structure–activity relationship of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J Med Chem 48:5215–5223
Acknowledgments
Authors thank Head—Department of Chemistry, and Director—CFRD, Osmania University, for providing facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna, C., Bhargavi, M.V., Rao, C.P. et al. Synthesis and antimicrobial assessment of novel coumarins featuring 1,2,4-oxadiazole. Med Chem Res 24, 3743–3751 (2015). https://doi.org/10.1007/s00044-015-1399-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1399-4