Abstract
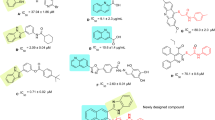
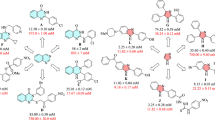
To develop a lead anti-diabetic compound, a series of 21 novel quinazoline derivatives have been synthesized and screened against α-glucosidase. The binding mode of the compounds at the active site of α-glucosidase was explored using Glide docking method. The binding model suggests one to four hydrogen bonding interactions between quinazoline derivatives and α-glucosidase. 6-Bromo-2-cyclopropyl quinazoline-4(3H)-one has been modified by C–C cross coupling to obtain nine different aryl scaffolds. These scaffolds further modified at C-4 position using amidation method to generate 21 compounds. Based on the interaction profile and docking score, all these compounds were selected for in vitro enzymatic screening. Seven of the thirty six compounds showed <20 µM activity against α-glucosidase and among these, compound 6f showed the highest inhibition, with an IC50 of 3.4 µM. In silico analysis was utilized to evaluate the diversity of the set of compounds against shape space, and relevant drug-like properties.











Similar content being viewed by others
References
Bridges AJ (2001) Chemical inhibitors of protein kinases. Chem Rev 101:2541–2572
Chandrika PM, Yakaiah T, Narsaiah B, Sridhar V, Venugopal G, Rao JV, Kumar KP, Murthy USN, Rao ARR (2009) Synthesis leading to novel 2, 4, 6-trisubstituted quinazoline derivatives, their antibacterial and cytotoxic activity against THP-1, HL-60 and A375 cell lines. Indian J Chem 48B:840–847
Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP (1997) Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J Comput-Aided Mol Des 11:425–445
Ferreres F, Gil-Izquierdo A, Vinholes J, Silva ST, Valentao P, Andrade PB (2012) Bauhinia forficata Link authenticity using flavonoids profile: relation with their biological properties. Food Chem 134:894–904
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Garlapati R, Pottabathini N, Gurram V, Chaudhary AB, Chunduri VR, Patro B (2012) Pd-catalyzed amination of 6-halo-2-cyclopropyl-3-(pyridyl-3-ylmethyl) quinazolin-4(3H)-one. Tetrahedron Lett 53:5162–5166
Garlapati R, Pottabathini N, Gurram V, Kasani KS, Gundla R, Thulluri C, Machiraju PK, Chaudhary AB, Addepally U, Chunduri VR, Patro B (2013) Development of α Glucosidase inhibitors by room temperature C–C cross couplings of quinazolinones. Org Biomol Chem 11:4778–4791
Hartwig JF (1998) Transition metal catalyzed synthesis of arylamines and aryl ethers from aryl halides and triflates: scope and mechanism. Angew Chem Int Ed 37:2046–2067
He Y, Wang X-B, Fan B-Y, Kong L-Y (2014) Honokiol trimers and dimers via biotransformation catalyzed by Momordica charantia peroxidase: novel and potent α-glucosidase inhibitors. Bioorg Med Chem 22:762–771
Kakkar T, Boxenbaum H, Mayersohn M (1999) Estimation of K i in a competitive enzyme-inhibition model: comparisons among three methods of data analysis. Drug Metab Dispos 27:756–762
Kato A, Hayashi E, Miyauchi S, Adachi I, Imahori T, Natori Y, Yoshimura Y, Nash RJ, Shimaoka H, Nakagome I, Koseki J, Hirono S, Takahata H (2014) α-1-C-butyl-1,4-dideoxy-1,4-imino-l-arabinitol as a second-generation iminosugar-based oral α-glucosidase inhibitor for improving postprandial hyperglycemia. J Med Chem 55:10347–10362
Lakshman MK, Frank J (2009) A simple method for C-6 modification of guanine nucleosides. Org Biomol Chem 7:2933–2940
Lakshman MK, Keeler JC, Hilmer JH, Martin JQ (1999) Palladium-catalyzed C–N bond formation: facile and general synthesis of N6-aryl 2′-deoxyadenosine analogues. J Am Chem Soc 121:6090–6091
Lakshman MK, Choudhury A, Bae S, Rochttis E, Pradhan P, Kumar A (2009) Synthesis of N6, N6-Dialkyladenine nucleosides using hexaalkylphosphorustriamides produced in situ. Eur J Org Chem 1:152–159
Liao JJ–L (2007) Molecular recognition of protein kinase binding pockets for design of potent and selective kinase inhibitors. J Med Chem 50:409–424
Long S, Stefani FR, Biondi S, Ghiselli G, Panunzio M (2013) N-heteroarylmethyl-5-hydroxy-1,2,5,6-tetrahydropyridine-3-carboxylic acid a novel scaffold for the design of uncompetitive α-glucosidase inhibitors. Bioorg Med Chem 21:5811–5822
Park H, Hwang KY, Oh KH, Kim YH, Lee JY, Kim K (2008) Discovery of novel α-glucosidase inhibitors based on the virtual screening with the homology-modeled protein structure. Bioorg Med Chem 16:284–292
Ram VJ, Farhanullah BrajendraK, Tripathi SrivastavaAK (2003) Synthesis and antihyperglycemic activity of suitably functionalized 3H-quinazolin-4-ones. Bioorg Med Chem 11:2439–2444
Rosenthal AS, Tanega C, Shen M, Mott BT, Bougie JM, Nguyen D-T, Misteli T, Auld DS, Maloney DJ, Thomas CJ (2011) Potent and selective small molecule inhibitors of specific isoforms of Cdc2-like kinases (Clk) and dual specificity tyrosine-phosphorylation-regulated kinases (Dyrk). Bioorg Med Chem Lett 21:3152–3158
Rudolph J, Esler WP, Connor SO, Coish PDG, Wickens PL, Brands M, Bierer DE, Bloomquist BT, Bondar G, Chen L, Chuang C-Y, Claus TH, Fathi Z, Fu W, Khire UR, Kristie JA, Liu X-G, Lowe DB, McClure AC, Michels M, Ortiz AA, Ramsden PD, Schoenleber RW, Shelekhin TE, Vakalopoulos A, Tang W, Wang L, Yi L, Gardell SJ, Livingston JN, Sweet LJ, Bullock WH (2007) Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. J Med Chem 50:5202–5216
Surber BW, Cross JL, Hannick SM (2010) Tenth international symposium on the synthesis and applications of isotopes and isotopically labelled compounds—poster presentations Session 19. J Label Compd Radiopharm 53:468–670
Sauer WHB, Schwarz MK (2003) Molecular shape diversity of combinatorial libraries: a prerequisite for broad bioactivity. J Chem Inf Model 43:987–1003
Van Brocklin HF, Lim JK, Coffing SL, Hom DL, Negash K, Ono MY, Gilmore JL, Bryant I, Riese DJ II (2005) Anilinodialkoxyquinazolines: screening epidermal growth factor receptor tyrosine kinase inhibitors for potential tumor imaging probes. J Med Chem 48:7445–7456
Wan ZK, Wacharasindhu S, Binnun E, Mansour T (2006) An efficient direct amination of cyclic amides and cyclic ureas. Org Lett 8:2425–2428
Wan ZK, Wacharasindhu S, Levins CG, Lin M, Tabei K, Mansour TS (2007) The scope and mechanism of phosphonium-mediated SNAr reactions in heterocyclic amides and ureas. J Org Chem 72:10194–10210
Wang Q-Q, Cheng N, Yi W-B, Peng S-M, Zou X-Q (2014) Synthesis, nitric oxide release, and α-glucosidase inhibition of nitric oxide donating apigenin and chrysin derivatives. Bioorg Med Chem 22:1515–1521
Wissner A, Floyd MB, Johnson BD, Fraser H, Ingalls C, Nittoli T, Dushin RG, Discafani C, Nilakantan R, Marini J, Ravi M, Cheung K, Tan X, Musto S, Annable T, Siegel MM, Loganzo F (2005) 2-(Quinazolin-4-ylamino)-[1,4]benzoquinones as covalent-binding, irreversible inhibitors of the kinase domain of vascular endothelial growth factor receptor-2. J Med Chem 48:7560–7581
Zhang M, Cui X, Chen X, Wang L, Li J, Wu Y, Hou L, Wu Y (2012) Facile synthesis of aryl(het)cyclopropane catalyzed by palladacycle. Tetrahedron 68:900–905
Acknowledgments
Authors are grateful to GVK Biosciences Pvt. Ltd., for the financial support and encouragement. We thank Prof. Mahesh K. Lakshman, Department of Chemistry, The City College and The City University of New York, New York, USA, for his invaluable support and motivation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gurram, V., Garlapati, R., Thulluri, C. et al. Design, synthesis, and biological evaluation of quinazoline derivatives as α-glucosidase inhibitors. Med Chem Res 24, 2227–2237 (2015). https://doi.org/10.1007/s00044-014-1293-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1293-5