Abstract
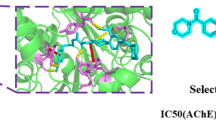
Acetylcholinesterase inhibitors (AChEIs) are currently the best available pharmacotherapy for Alzheimer patients, but because of bioavailability issues, there is still great interest in discovering better AChEIs. The aporphine alkaloid is an important class of natural products, which shows diverse biological activity, such as acetylcholinesterase inhibitory activity. To find new lead AChEIs compounds, eight aporphine alkaloids were synthesized by O-dealkylation, N-dealkylation, and ring aromatization reactions using nuciferine as raw material. The anti-acetylcholinesterase activity of synthesized compounds was measured using modified Ellman’s method. The results showed that some synthesized compounds exhibited higher affinity to AChE than the parent compound nuciferine. Among these compounds, 1,2-dihydroxyaporphine (2) and dehydronuciferine (5) were the most active compounds (IC50 = 28 and 25 μg/mL, respectively). Preliminary analysis of structure–activity relationships suggested that aromatization of the C ring, the presence of the alkoxyl group at C1 and the hydroxy group at C2 position as well as the alkyl substituent at the N atom were favorable to the acetylcholinesterase inhibition. Molecular docking was also applied to predict the binding modes of compounds 1, 2, and 9 into the huperzine A binding site of AChE.









Similar content being viewed by others
References
Acosta K, Cessac JW, Rao PN, Kim HK (1994) Oxidative demethylation of 4-substituted N,N-dimethylanilines with iodine and calcium-oxide in the presence of methanol. J Chem Soc 17:1985–1986
Ahmad R, Saa JM, Cava MP (1977) Regioselective O-demethylation in aporphine alkaloid series. J Org Chem 42:1228–1230
Barolo SM, Teng X, Cuny GD, Rossi RA (2006) Syntheses of aporphine and homoaporphine alkaloids by intramolecular ortho-arylation of phenols with aryl halides via S(RN)1 reactions in liquid ammonia. J Org Chem 71:8493–8499
Boustie J, Stigliani JL, Montanha J, Amoros M, Payard P, Girre L (1998) Antipoliovirus structure–activity relationships of some aporphine alkaloids. J Nat Prod 61:480–484
Cava MP, Veznkatfswarlu A, Srinivasa M, Edie DL (1972) Oxidative tranformations in the aporphine alkaloid series. Tetrahedron 28:4299–4307
Couture A, Deniau E, Woisel P, Grandclaudon P, Carpentier JF (1996) Base-induced cyclization of trimethoxy-O-aroyldiphenylphosphoryl methylbenz-amide: A formal synthesis of (+/−) cherylline and (+/−) cherylline dimethylether. Tetrahedron Lett 37:3697–3700
Guinaudeau H, Leboeuf M, Cave A (1979) Aporphine alkaloids. II. J Nat Prod 42:325–360
Guinaudeau H, Leboeuf M, Cave A (1983) Aporphinoid alkaloids III. J Nat Prod 46:761–835
Han BH, Park MH, Han YN (1989) Aporphine and tetrahydrobenzylisoquin-oline alkaloids from the seeds of Zizyphus vulgaris var. spinosus. Arch Pharm Res 12:263–268
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Horie T, Tominaga H, Kawamura Y, Yamada T (1992) Studies of the selective O-Alkylation and dealkylation of flavonoids. 13. An improved method for synthesizing 5,6,7-trihydroxyflavones from 6-hydroxy-5,7-dimet-hoxyflavones. J Org Chem 57:3343–3347
Horie T, Kobayashi T, Kawamura Y, Yoshida I, Tominaga H, Yamashita K (1995) Studies of the selective O-alkylation and dealkylation of flavonoids.XVIII. A convenient method for synthesizing 3,5,6,7-tetradroxyflavones. Bull Chem Soc Jpn 68:2033–2041
Huang WJ, Chen CH, Singh OV, Lee SL, Lee SS (2002) A facile method for the synthesis of glaucine and norglaucine from boldine. Syn Commun 32:3681–3686
Kelly PH, Miller RJ, Neumeyer JL (1976) Aporphines. 16. Action of aporphine alkaloids on locomotor activity in rats with 6-hydroxydopamine lesions of the nucleus accumbens. Eur J Pharm 35:85–92
Kuo RY, Chang FR, Chen CY, Teng CM, Yen HF, Wu YC (2001) Antiplatelet activity of N-methoxycarbonyl aporphines from Rollinia mucosa. Phytochemistry 57:421–425
Lahiri DK, Farlow MR, Greig NH, Sambamurti K (2002) Current drug targets for Alzheimer’s disease treatment. Drug Dev Res 56:267–281
Lu ST, Wu YC, Leou SP (1987) The oxidation of isoquinoline alkaloids with m-chloroperbenzoic acid. J Chin Chem Soc (Taipei, Taiwan) 34:33–42
Markmee S, Ruchirawat S, Prachyawarakorn V, Ingkaninan K, Khorana N (2006) Isoquinoline derivatives as potential acetylcholinesterase inhibitors. Bioorg Med Chem Lett 16:2170–2172
Munoz TD, Camps P (2006) Dimeric and hybrid anti-Alzheimer drug candidates. Curr Med Chem 13:399–422
Racchi M, Mazzucchelli M, Porrello E, Lanni C, Govoni S (2004) Acetylcholinesterase inhibitors: novel activities of old molecules. Pharmacol Res 50:441–451
Rollinger JM, Schuster D, Baier E, Ellmerer EP, Langer T, Stuppner H (2006) Taspine: bioactivity-guided isolation and molecular ligand-target insight of a potent acetylcholinesterase inhibitor from Magnolia soulangiana. J Nat Prod 69:1341–1346
Scarpini E, Scheltens P, Feldman H (2003) Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol 2:539–547
Wafo P, Nyasse B, Fontaine C, Sondengam BL (1999) Aporphine alkaloids from Enantia chlorantha. Fitoterapia 70:157–160
Wang LL, Liu B, Shi RB (2009) Study on chemical constituents of Folium Nelumbinis. Nat Prod Res Dev 21:416–419
Yang ZD, Zhang X, Du J, Ma ZJ, Guo F, Li S, Yao XJ (2012) An aporphine alkaloid from Nelumbo nucifera as an acetylcholinesterase inhibitor and the primary investigation for structure–activity correlations. Nat Prod Res 26:387–392
Zou CL, Ji H, Xie GB, Chen DL, Wang FP (2008) An effective O-demethylation of some C19-diterpenoid alkaloids with HBr–glacial acetic acid. J Asian Nat Prod Res 10:1063–1067
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (No. 21262022), the Elitist Program of Lanzhou University of Technology (No. J201303) and Zhejiang Provincial Natural Science Foundation of China (No. LY12B02005). The authors also thank Miss Karen Tenney, a researcher of University of California, Santa Cruz, for revising the grammar errors
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Z., Song, Z., Xue, W. et al. Synthesis and structure–activity relationship of nuciferine derivatives as potential acetylcholinesterase inhibitors. Med Chem Res 23, 3178–3186 (2014). https://doi.org/10.1007/s00044-013-0905-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0905-9