Abstract
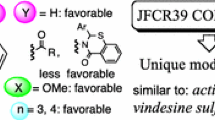
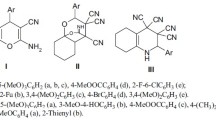
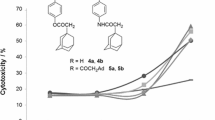
The research article describes the effect of an ester group on the in vitro antiproliferative activity in SAR studies of 5-methyl-5H-indolo[2,3-b]quinoline (neocryptolepine) derivatives. The C-2 and/or C-9 ester-substituted neocryptolepines were synthesized starting from indole-3-carboxylates and N-methylanilines, which were bearing an ester group. To these ester-substituted neocryptolepines, various aminoalkylamino substituents were further attached at the C-11, and an in vitro antiproliferative assay was performed by varying the substituents at the C-11 and the position of the ester group in the A and/or D ring of neocryptolepines. Results indicated that the antiproliferative activities of the agents could be improved by introducing an ester substituent at the C-9 position. Among them, the methyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate (8b) was the most potent agent with an IC50 value of 0.044 μM against the human leukemia MV4-11 cell line. The selective cytotoxicity of the agents between the cancer cell lines and normal cell lines were also described. The antiproliferative potency of dimethyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-2,9-dicarboxylate (9a) against the human colon cancer cell line HCT116 is 28 times higher than against the normal mice fibroblast cell line BALB/3T3.
Graphical Abstract





Similar content being viewed by others
References
Alexandra P, Elsa TG, Jonathan S, Dave CW, Peter JH (2000) Antiplasmodial activity of cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Med 66:30–34
Bailly C, Laine W, Baldeyrou B, De Pauw-Gillet MC, Colson P, Houssier C, Cimanga K, Van Miert S, Vlietinck AJ, Pieters L (2000) DNA intercalation, topoisomerase II inhibition and cytotoxic activity of the plant alkaloid neocryptolepine. Anti Canc Drug Des 15:191–201
Cimanga K, De Bruyne T, Lasure A, Van poel B, Pieters L, Claeys M, Vanden Berghe D, Kambu K, Tona L, Vlietinck AJ (1996a) In vitro biological activities of alkaloids from cryptolepis sanguinolenta. Planta Med 62:22–27
Cimanga K, De Bruyne T, Pieters L, Claeys M, Vlietinck A (1996b) New alkaloids from Cryptolepis sanguinolenta. Tetrahedron Lett 37:1703–1706
Cimanga K, De Bruyne T, Pieters L, Vlietinck AJ, Turger CA (1997) In vitro and in vivo antiplasmodial activity of cryptolepine and related alkaloids from cryptolepis sanguinolenta. J Nat Prod 60:688–691
Cimanga K, De Bruyne T, Pieters L, Totte J, Tona L, Kambu K, Vanden Berghe D, Vlietinck AJ (1998) Antibacterial and antifungal activities of neocryptolepine, biscryptolepine, and cryptoquindoline, alkaloids isolated from Cryptolepis sanguinolenta. Phytomedicine 5:209–221
Guittat L, Alberti P, Rosu F, Van Miert S, Thetiot E, Pieters L, Gabelica V, De Pauw E, Ottaviani A, Riou JF, Mergny JL (2003) Interactions of cryptolepine and neocryptolepine with unusual DNA structures. Biochimie 85:535–547
Jonckers THM, Van Miert S, Cimanga K, Bailly C, Colson P, De Pauw-Gillet MC, van den Heuvel H, Claeys M, Lemiere F, Esmans EL, Rozenski J, Quirijnen L, Maes L, Dommisse R, Lemiere GLF, Vlietinck A, Pieters L (2002) Synthesis, cytotoxicity, and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. J Med Chem 45:3497–3508
Kirby GC, Paine A, Warhurst DC, Noamese BK, Phillipson JD (1995) In vitro and in vivo antimalarial activity of cryptolepine, a plant-derived indoloquinoline. Phytother Res 9:359–363
Kumar EVKS, Etukala JR, Ablordeppey SY (2008) Indolo[3,2-b]quinolines: synthesis, biological evaluation and structure activity-relationships. Mini-Rev in Med Chem 8:538–554
Lavrado J, Moreira R, Paulo A (2010) Indoloquinolines as scaffolds for drug discovery. Curr Med Chem 17:2348–2370
Linton EC, Kozlowski MC (2008) Catalytic enantioselective meerwein-eschenmoser claisen rearrangement: asymmetric synthesis of allyl oxindoles. J Am Chem Soc 130:16162–16163
Ojima I, Kuduk SD, Pera P, Veith JM, Bernacki RJ (1997) Synthesis and structure-activity relationships of nonaromatic taxoids: effects of alkyl and alkenyl ester groups on cytotoxicity. J Med Chem 40:279–285
Parvatkar PT, Parameswaran PS, Tilve SG (2011) Isolation, biological activities, and synthesis of indoloquinoline alkaloids: cryptolepine, isocryptolepine, and neocryptolepine. Curr Org Chem 15:1036–1057
Philippe G, Lobo R, Valerie M, Eric D, Joseph S, Francois F, Francois T, Bernard B, Jean-Louis P (1996) Antimalarial activity of cryptolepine and isocryptolepine, alkaloids isolated from Cryptolepis sanguinolenta. Phytother Res 10:317–321
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Wang L, Switalska M, Mei ZW, Lu WJ, Takahara Y, Feng XW, El-Sayed IE, Wietrzyk J, Inokuchi T (2013) Synthesis and in vitro antiproliferative activity of new 11-aminoalkylamino-substituted 5H- and 6H-indolo[2,3-b]quinolines; structure-activity relationships of neocryptolepines and 6-methyl congeners. Bioorg Med Chem 20:4820–4829
Wang L, Lu WJ, Odawara T, Misumi R, Mei ZW, Peng W, El-Sayed IE, Inokuchi T (2013) Improved synthesis and reaction of 11-chloroneocryptolepines, strategic scaffold for antimalaria agent, and their 6-methyl congener fromindole-3-carboxylate. J Heterocyclic Chem 49 (in press)
Wender PA, Hinkle KW (2000) Synthesis and biological evaluation of a new class of bryostatin analogues: the role of the C20 substituent in protein kinase C binding. Tetrahedron Lett 41:6725–6729
Wright CW, Phillipson JD, Awe SO, Kirby GC, Warhurst DC, Quetin-Leclercq J, Angenot L (1996) Antimalarial activity of cryptolepine and some other anhydronium bases. Phytother Res 10:361–363
Xiong J, Zhu HF, Zhao YJ, Lan YJ, Jiang JW, Yang JJ, Zhang SF (2009) Synthesis and antitumor activity of amino acid ester derivatives containing 5-fluorouracil. Molecules 14:3142–3152
Yang LX, Pan XD, Wang HJ (2002) Novel camptothecin derivatives. Part 1: oxyalkanoic acid esters of camptothecin and their in vitro and in vivo antitumor activity. Bioorg Med Chem Lett 12:1241–1244
Acknowledgments
We gratefully acknowledge supports by Okayama University and the Advanced Science Research Center for NMR and EA. We thank MEXT for the scholarship to L.W. We are thankful to Prof. S. Nakashima and Prof. X.-Q.Yu, Sichuan University, for HRMS analyses, and to Prof. J. Futami for UV measurements. Support by Adaptable and Seamless Technology Transfer Program of JST and generous gift of various indoles from Air Water Inc. are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, WJ., Świtalska, M., Wang, L. et al. In vitro antiproliferative activity of 11-aminoalkylamino-substituted 5H-indolo[2,3-b]quinolines; improving activity of neocryptolepines by installation of ester substituent. Med Chem Res 22, 4492–4504 (2013). https://doi.org/10.1007/s00044-012-0443-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0443-x