Abstract
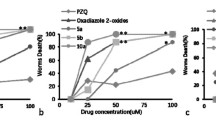
A set of aminosubstituted neocryptolepine (5-methyl-5H-indolo[2,3-b]quinoline) derivatives has been synthesized and evaluated for in vitro activity against Schistosoma mansoni adult worms. Six out of 18 derivatives showed significant antischistosomal activity with 100% worm mortality at a concentration of 5 μg/ml after 5 days. The most effective compound is neocryptolepine 10d having IC50 and IC90, 2.70 and 3.95 μM/ml for Egyptian Schistosoma strains, respectively. It is clear from the results that introducing N-substituted side-chains into the indoloquinoline core structure significantly increased the antischistosomicidal activity. The structure–activity relationships of this series of compounds are discussed.
Graphical Abstract
Neocryptolepine derivatives containing a basic side-chain at position C-11 were synthesized and evaluated against Egyptian Schistosoma strains. The most active compound 10d showed a significant antischistosomal activity with 100% worm mortality and IC50 of 2.70 μM/ml.




Similar content being viewed by others
References
Alajarin M, Molina P, Vidal A (1997) Formal total synthesis of the alkaloid cryptotackieine (neocryptolepine). J Nat Prod 60:747–748
Botros S, Bennett J (2007) Praziquantel resistance. Expert Opin Drug Discov 2:535–540
Doenhoff MJ, Pica-Mattoccia L (2006) Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev Anti-Infect Ther 4:199–210
Doenhoff M, Kusel JR, Coles GC, Cioli D (2002) Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Parasitol 96:465–469
El Sayed I, Van der Veken P, Dhooghe L, Hostyn S, Van Baelen G, Lemière G, Maes BUW, Cos P, Maes L, Joossens J, Haemers A, Pieters L, Augustyns K (2009) Synthesis and antiplasmodial activity of aminoalkylamino substituted neocryptolepine derivatives. J Med Chem 52:2979–2988
Fenwick A, Webster JP (2006) Schistosomiasis: challenge for control, treatment and drug resistance. Curr Opin Infect Dis 19:577–582
Gonnert R, Andrew P (1977) Praziquantel, a new board-spectrum antischistosomal agent. Z Parasitenkd 52:129–150
Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL (1999) Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg 60:932–935
Jonckers TH, van Miert S, Cimanga K, Bailly C, Colson P, De Pauw-Gillet MC, van den Heuvel H, Claeys M, Lemière F, Esmans EL, Rozenski J, Quirijnen L, Maes L, Dommisse R, Lemière GL, Vlietinck A, Pieters L (2002) Synthesis, cytotoxicity and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. J Med Chem 45:3497–3508
Parvatkar TP, Parameswaran SP, Tilve GS (2011) Isolation, biological activities and synthesis of indoloquinoline alkaloids: cryptolepine, isocryptolepine and neocryptolepine. Curr Org Chem 15:1036–1057
Ramirez B, Bickle Q, Yousif F, Mouries MA, Nwaka S (2007) Schistosom challenge in compound screening. Expert Opin Drug Discov 2(S1):1–9
Shi C, Zhang Q, Wang KJ (1999) Biradicals from thermolysis of N-[2-(1-alkynyl)phenyl]-N′-phenylcarbodiimides and their subsequent transformations to 6H-indolo[2,3-b]quinolines. J Org Chem 64:925–932
Southgate VR, Rollinson D, Tchuen Tchuente A, Hagan P (2005) Towards control of schistosomiasis in sub-Saharan Africa. J Helminthol 79:181–185
WHO (2011) http://www.who.int/schistosomiasis/epidemiology/en/, updated Feb 2011
Wright CW (2005) Plant derived antimalarial agents: new leads and challenges. Phytochem Rev 4:55–61
Yousif F, Hifnawy MS, Soliman G, Boulos L, Labib Th, Mahmoud S, Ramzy F, Yousif M, Hassan I, Mahmoud K, El-Hallouty SM, EL-Gendy M, Gohar L, EL-Manawaty M, EL-Menshawi BS (2007) Large-scale in vitro screening of Egyptian native and cultivate plants for schistosomicidal activity. Pharma Biol 45:501–510
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sayed, I.E., Ramzy, F., William, S. et al. Neocryptolepine analogues containing N-substituted side-chains at C-11: synthesis and antischistosomicidal activity. Med Chem Res 21, 4219–4229 (2012). https://doi.org/10.1007/s00044-011-9934-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9934-4