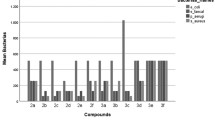
Abstract
Various 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles (11–20) were prepared by the reaction of aryl substituted hydrazones of 4-fluorobenzoic acid hydrazide (1–10) with acetic anhydride. The structures of the synthesized compounds 11–20, were confirmed by UV, IR, 1H-NMR and mass spectroscopic methods. Antifungal evaluation of the hydrazide–hydrazones 1–10 and corresponding 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles 11–20, against clinical and standard Candida pathogens have been performed by using agar diffusion to indentify the active compounds, which were later subjected to a broth microdilution assay to justify the activity level in terms of minimum inhibitory concentrations (MIC). 4-Fluorobenzoic acid [(5-bromothiophen-2-yl)methylene]hydrazide, showed the highest inhibitory activity against Candida albicans (MIC: 125 μg/ml), and when compared with ketoconazole. In addition, bioauthographic antifungal activity against plant pathogenic fungi such as Colletotrichum, Botrytis, Fusarium, and Phomopsis was conducted. 4-Fluorobenzoic acid [(5-bromothiophen-2-yl)methylene]hydrazide was the most active analog against P. viticola with 91% inhibition at 30 μM after 144 h. Furthermore, known and the newly synthesized compounds were also screened through a panel of bioassays to determine their anti-inflammatory, cytotoxic, and antioxidant activities in mammalian cells. 3-Acetyl-5-(4-fluorophenyl)-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole, showed a strong inhibition of NF-κB-dependent transcription in SW1353 cells with IC50 value of 0.75 μg/ml. 4-Fluorobenzoic acid [(3-hydroxy-4-methoxyphenyl)methylene]hydrazide, and 3-acetyl-5-(4-fluorophenyl)-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole on intracellular ROS generation in PMA induced HL-60 cells demonstrated potent activity with IC50 values of 0.9 μg/ml. A strong inhibition of the activity of iNOS activity in LPS induced RAW 264.7 cells was observed for 3-acetyl-5-(4-fluorophenyl)-2-(4-hydroxyphenyl)-2,3-dihydro-1,3,4-oxadiazole with IC50 value of 0.3 μg/ml.






Similar content being viewed by others
References
Ankisetty S, Gochfeld DJ, Diaz MC, Khan SI, Slattery M (2010) Chemical constituents of the deep reef Caribbean sponges Plakortis angulospiculatus and Plakortis halichondrioides and their anti-inflammatory activities. J Nat Prod 73:1494–1498
Durgun B, Capan G, Ergenc N, Rollas S (1993) Synthesis, characterization and biological evaluation of new benzylidenebenzohydrazides and 2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles. Pharmazie 48:942–943
Ergenc N, Rollas S, Topaloglu Y, Otuk G (1989) Synthesis andcharacterization of new 1,3,4-oxadiazolines. Arch Pharm (Weinheim) 322:837–838
Hassan GS, Farag NA, Hegazy GH, Arafa RK (2008) Design and synthesis of novel benzopyran-2-one derivatives of expected antimicrobial activity through DNA gyrase-B inhibition. Arch Pharm Chem Life Sci 341:725–733
Jin L, Chen J, Song B, Chen Z, Yang S, Li Q, Hu D, Xu R (2006) Synthesis, structure, and bioactivity of N0-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg Med Chem Lett 16:5036–5040
Khalil AA, Hamide SGA, Al-Obaid AM, El-Subbagh HI (2003) Substituted quinazolines, Part 2. Synthesis and in vitro anticancer evaluation of new 2-substituted mercapto-3H-quinazoline analogs. Arch Pharm Pharm Med Chem 2:95–103
Koçyigit-Kaymakcıoglu B, Emre-Oruc E, Unsalan S, Rollas S (2009) Synthesis and antituberculosis activity of hydrazide-hydrazones. Med Chem Res 118:277–286
Koçyigit-Kaymakcıoglu B, Oruc E, Unsalan S, Kandemirli F, Shvets N, Rollas S, Dimoglo A (2006) Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity. Eur J Med Chem 41:1253–1261
Li S, Luo Y, Wang L, Li D (2010) Synthesis of 1,3,4-oxazolines and 1,3,4-oxadiazole containing fluorine and their anticancer activity. Hecheng Huaxue 18:611–613 (Chem. Abst. 2010, 154:336040)
NCCLS, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, second ed., NCCLS document M27-A2 [ISBN 1-56238-469-4], 2002
Özdemir A, Turan-Zitouni G, Kaplancıklı ZA, İşcan G, Khan S, Demirci F (2010) Synthesis and the selective antifungal activity of 5,6,7,8-tetrahydroimidazo[1,2-a]pyridine derivatives. Eur J Med Chem 45:2080–2084
Quang DN, Harinantenaina L, Nishizawa T, Hashimoto T, Kohchi C, Soma G, Asakawa Y (2006) Inhibition of nitric oxide production in RAW 264.7 cells by azaphilones from Xylariaceous fungi. Biol Pharm Bull 29:34–37
Rollas S, Gulerman N, Erdeniz H (2002) Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 57:171–174
Shirote PJ, Bhatia MS (2011) Synthesis and goat pulmonary vasodilatory activity of some novel 1,3,4-oxadiazoles. Arab J Chem 4:413–418
Sobolev VS, Khan SI, Tabanca N, Wedge DE, Manly SP, Cutler SJ, Coy MR, Becnel JJ, Neff SA, Gloer JB (2011) Biological activity of peanut (Arachis hypogaea) phytoalexins and selected natural and synthetic Stilbenoids. J Agric Food Chem 59:1673–1682
Steel RGD, Torrie JH (1980) Principles and procedures of statistics: a biometrical approach, 2nd edn. McGraw Hill Book Company, New York
Tabanca N, Bedir E, Kirimer N, Baser KHC, Khan SI, Jacob MR, Khan IA (2003) Antimicrobial Compounds from Pimpinella Species Growing in Turkey. Planta Med 69:933–938
Tabanca N, Khan SI, Ma G, Bedir E, Pasco DS, Kirimer N, Baser KHC, Khan IA (2007a) Effect of essential oils and isolated compounds from Pimpinella species on NF-kappaB: a target for antiinflammatory therapy. Phytother Res 21:741–745
Tabanca N, Pawar RS, Ferreira D, Marais JPJ, Khan SI, Joshi V, Wedge DE, Khan IA (2007b) Flavan-3-ol-phenylpropanoid conjugates from Anemopaegma arvense and their antioxidant activities. Planta Med 73:1107–1111
Wedge DE, Camper ND (2000) Connections between agrochemicals and pharmaceuticals. In: Cutler SJ, Cutler HG (eds) Biological active natural products: pharmaceuticals. CRC press LLC, New York, pp 1–15
Wedge DE, Kuhajek JM (1998) A microbioassay for fungicide discovery. SAAS Bull Biochem Biotech 11:1–7
Acknowledgments
This project was financially supported by the Marmara University Research Center (Project number SAG-060-210). The authors express their gratitude for USDA, ARS, NPURU financial support. Also Ms. J. Linda Robertson, Ms. Ramona Pace, and Ms. Xiaoning Wang for assistance with the antifungal assay, Mr. John Trott, Mr. Paul Bates, and Ms. Katherine Martin for bioassays for anti-inflammatory, antioxidant, and cytotoxic activities are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Koçyiğit-Kaymakçıoğlu, B., Oruç-Emre, E.E., Ünsalan, S. et al. Synthesis and biological activity of hydrazide–hydrazones and their corresponding 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles. Med Chem Res 21, 3499–3508 (2012). https://doi.org/10.1007/s00044-011-9882-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9882-z