Abstract
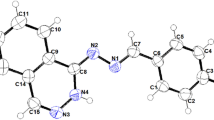
New transition metal complexes of quinoxaline–thiosemicarbazone ligands were prepared and characterised by spectroanalytical techniques. The ligands L1H2 and L2H2 were obtained by the reaction of quinoxaline-2.3(1,4H)-dione with methyl and phenyl thiosemicarbazide, respectively. All the complexes are found to be monomeric in nature and have tetrahedral geometry. The copper complexes have shown redox responses in the applied voltage range, whereas the ligands and other complexes are electrochemically innocent. The ligands, copper and zinc complexes are explored for antidiabetic activity in the diabetes-induced Wister rats. Evaluation of antidiabetic activity was done by blood-glucose test and oral glucose tolerance test; few compounds have exhibited significant antidiabetic activity and posses low toxicity with a high safety profile.



Similar content being viewed by others
References
Badran MM, Abouzid KAM, Hussein MHM (2003) Synthesis of certain substituted quinoxalines as antimicrobial agents (part II). Arch Pharm Res 26:107–113
Bailar JC, Emeleus HJ, Nyholm SR, Dickenson AFT (1975) Comprehensive inorganic chemistry. Pergamon Press, New York, p 3
Breslin HJ, Miskowski TA, Kukla MJ, De Winter HL, Somers MVF, Roevens PWM, Kavash RW (2003) Tripeptidyl-peptidase II (TPP II) inhibitory activity of (S)-2,3-dihydro-2-(1H-imidazol-2-yl)-1H-indoles, a systematic SAR evaluation. Part 2. Bioorg Med Chem Lett 13(24):4467–4471
Das U, Pati HN, Panda AK, De Clerc E, Balzarini J, Molnár J, Baráth Z, Ocsovszki I, Kawase M, Zhou L, Sakagami H, Dimmock JR (2009) 2-(3-Aryl-2-propenoyl)-3-methylquinoxaline-1,4-dioxides: a novel cluster of tumor-specific cytotoxins which reverse multidrug resistance. Bioorg Med Chem 17:3909–3915
Deleuze-Masquefa C, Moarbess G, Khier S, David N, Gayraud-Paniagua S, Bressolle F, Pinguet F, Bonnet PA (2009) New imidazo [1, 2-a]quinoxaline derivatives: synthesis and in vitro activity against human melanoma. Eur J Med Chem 44:3406–3411
Dudash J Jr, Zhang Y, Moore JB, Look R, Liang Y, Beavers MP, Conway BR, Rybczynski PJ, Demarest KT (2005) Synthesis and evaluation of 3-anilino-quinoxalinones as glycogen phosphorylase inhibitors. Bioorg Med Chem Lett 15:4790–4793
Dutta RL, Syamal A (1993) Elements of magneto chemistry, 2nd edn. E. W. Press, New Delhi
Fosset M, De Weille JR, Green RD, Schmid-Antomarchi H, Lazdunski M (1988) Antidiabetic sulfonylureas control action potential properties in heart cells via high affinity receptors that are linked to ATP-dependent K+ channel. J Biol Chem 263:7933–7936
Geary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7:81–122
Kulkarni NV, Hegde GS, Kurdekar GS, Budagumpi S, Sathisha MP, Revankar VK (2010) Spectroscopy, electrochemistry, and structure of 3D-transition metal complexes of thiosemicarbazones with quinoline core: evaluation of antimicrobial property. Spectrosc Lett 43:235–246
Lever ABP (1968) Inorganic electronic spectroscopy. Elsevier Publishing Company, New York
Mayer G, Taberner PV (2002) Effects of the imidazoline ligands (±)-efaroxan and KU14R on blood glucose homeostasis in the mouse. Eur J Pharmacol 454:95–102
Mishra AK, Dandiya PC, Kulkarni SK (1973) Anticonvulsant activity of some trimethoxybenzylidene-2-thiohydantoin derivatives. Indian J Pharmacol 5:449–450
Moller DE (2001) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414:821–827
Naik AD, Annigeri SM, Gnagadharmath UB, Revankar VK, Mahale VB, Reddy VK (2002a) Anchoring mercapto-triazoles on dicarbonyl backbone to assemble novel binucleating, acyclic SNONS compartmental ligands. Indian J Chem 41A:2046–2053
Naik AD, Annigeri SM, Gangadharmath UB, Revankar VK, Mahale VB (2002b) Bimetallic complexes of a potentially pentadentate, acyclic, symmetrical compartmental Schiff base ligand that provides suitable topology for an exogenous bridge. Trans Met Chem 27:333–336
Nawrocka W (1996) Syntheses and pharmacological properties of new 2-aminobenzimidazole derivatives. Boll Chi Farm 135:18–23
O Neil MJ, Smith M, Heckelman PE (eds) (2001) The Merck Index, 13th ed, Merck & Co. Inc., New Jersey. Monograph Number, 10074, p 1785
Philips MA (1928) The formation of 2-substituted benziminazoles. J Chem Soc 2393–2399
Reddy Shastry CV, Marwah P, Shankar Rao G (1989) Synthesis and biological activity of some new N-aryl carbamoyl and aryl thiocarbamoyl hydrazinoquinoxalin-2-ones. Indian J Chem 28B:885–891
Sakurai H, Katoh A, Yoshikawa Y (2006) Chemistry and biochemistry of insulin mimeticvanadium and zinc complexes, trial for treatment of diabetes mellitus. Bull Chem Soc Jpn 79:1645–1664
Seleem HS, Shetary BAEL, Khalil SME, Mostafa M, Shebl M (2005) Structural diversity in copper(II) complexes of bis(thiosemicarbazone) and bis(semicarbazone) ligands. J Coord Chem 58:479–493
Sen AB, Gupta SK (1962) Possible antiamoebic agents part XIX. Synthesis of 1,3,4-thiadiazolines and di-1, 3,4-thiadiazolines. J Ind Chem Soc 39:628–634
Vogel AI (1961) A text book of quantitative inorganic analysis, 3rd edn. Longmans Green and Co., Ltd, London, p 266
Wright JB (1951) The chemistry of the benzimidazoles. Chem Rev 48:397–537
Yasumatsu N, Yoshikawa Y, Adachi Y, Sakurai H (2007) Antidiabetic copper(II)-picolinate: impact of the first transition metal in the metallopicolinate complexes. Bioorg Med Chem 15:4917–4922
Yoshikawa Y, Kondo M, Sakurai H, Kojima Y (2005) A family of insulinomimetic zinc(II) complexes of amino ligands with Zn(Nn) (n = 3 and 4) coordination modes. J Inorg Biochem 99:1497–1503
Yoshikawa Y, Hirata R, Yasui H, Sakurai H (2009) Alpha-glucosidase inhibitory effect of anti-diabetic metal ions and their complexes. Biochimie 91:1339–1341
Acknowledgments
The authors thank Department of Chemistry and USIC, Karnatak University, Dharwad for the spectral facility. Recording of FAB-mass spectra (CDRI Lucknow) are gratefully acknowledged. Further, the author (Naveen V. Kulkarni) thank Karnatak University, Dharwad for providing Nilekani fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kulkarni, N.V., Revankar, V.K., Kirasur, B.N. et al. Transition metal complexes of thiosemicarbazones with quinoxaline hub: an emphasis on antidiabetic property. Med Chem Res 21, 663–671 (2012). https://doi.org/10.1007/s00044-011-9576-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9576-6