Abstract
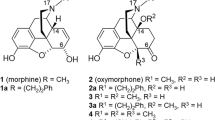
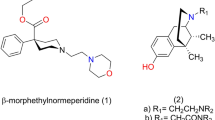
7α-Phenyl-6α,14α-endo-etheno-tetrahydrothebaine is an analogue of morphine but with much weaker affinity and efficacy to opioid receptors. Three compounds with o-, m- and p-amino substitution on its 7α-phenyl group were designed and synthesized to evaluate their κ-opioid receptor agonistic activity and antinociceptive effect. The introduction of amino group greatly increased the binding activity to κ-opioid receptor but only compound with p-substitution showed agonistic activity with potency similar to the marketed κ agonist butorphanol. In vivo antinociceptive test showed that compound with p-amino substitution displayed highest antinociceptive activity while compounds with o-, m-amino substitution showed partial or little analgesia effects. All these data indicate that p-amino substitution conferred κ agonist activity, suggesting that 7α-phenyl-6α,14α-endo-etheno-tetrahydrothebaines with a p-amino substitution may contain a novel pharmacophore component and could be considered as a leading compound for novel antinociceptive agents.





Similar content being viewed by others
References
Aldrich JV, McLaughlin JP (2009) Peptide kappa opioid receptor ligands: potential for drug development. AAPS J 11:312–322
Bentley KW, Hardy DG (1967) Novel analgesics and molecular rearrangements in the morphine-thebaine group. I. Ketones derived from derived from 6,14-endo-ethenotetrahydrothebaine. J Am Chem Soc 89:3267–3273
Bentley KW, Hardy DG, Meek B (1967) Novel analgesics and molecular rearrangements in the morphine-thebaine group. II. Alcohols derived from 6,14-endo-etheno- and 6,14-endo-ethanotetrahydrothebaine. J Am Chem Soc 89:3273–3280
Casy AF, Parfitt RT (1986) Opioid analgesics: chemistry and receptors. Plenum, New York, p 518
Christo PJ, Mazloomdoost D (2008) Cancer pain and analgesia. Ann N Y Acad Sci 1138:278–298
Coop A, Norton CL, Berzetei-Gurske I, Burnside J, Toll L, Husbands SM, Lewis JW (2000) Structural determinants of opioid activity in the orvinols and related structures: ethers of orvinol and isoorvinol. J Med Chem 43:1852–1857
Gallantine EL, Meert TF (2008) Antinociceptive and adverse effects of mu- and kappa-opioid receptor agonists: a comparison of morphine and U50488-H. Basic Clin Pharmacol Toxicol 103:419–427
Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD (1996) Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med 2:1248–1250
Gupta K, Vats ID, Gupta YK, Saleem K, Pasha S (2008) Lack of tolerance and morphine-induced cross-tolerance to the analgesia of chimeric peptide of Met-enkephalin and FMRFa. Peptides 29:2266–2275
Hamabe W, Yamane H, Harada S, Tokuyama S (2008) Involvement of kappa opioid receptors in the inhibition of receptor desensitization and PKC activation induced by repeated morphine treatment. J Pharm Pharmacol 60:1183–1188
Lewis JW, Readhead MJ (1973) Novel analgetics and molecular rearrangements in the morphine-thebaine group. 29. Aryl and arylalkyl tertiary alcohols in the 6,14-endo-ethenotetrahydrothebaine series. J Med Chem 16:84–85
Li W, Tang Y, Xie Q, Sheng W, Qiu ZB (2006) 3D-QSAR studies of orvinol analogs as kappa-opioid agonists. J Mol Model 12:877–884
Li W, Tao YM, Tang Y, Xu XJ, Chen J, Fu W, Wang XH, Chao B, Sheng W, Xie Q, Qiu ZB, Liu JG (2010) Highly selective and potent mu opioid ligands by unexpected substituent on morphine skeleton. Bioorg Med Chem Lett 20:418–421
McKnight AT, Corbett AD, Paterson SJ, Magnan J, Kosterlitz HW (1982) Comparison of in vitro potencies in pharmacological and binding assays after inhibition of peptidases reveals that dynorphin (1–9) is a potent kappa-agonist. Life Sci 31:1725–1728
Nagase H, Hayakawa J, Kawamura K, Kawai K, Takezawa Y, Matsuura H, Tajima C, Endo T (1998) Discovery of a structurally novel opioid kappa-agonist derived from 4,5-epoxymorphinan. Chem Pharm Bull (Tokyo) 46:366–369
Oka T, Negishi K, Suda M, Matsumiya T, Inazu T, Ueki M (1981) Rabbit vas deferens: a specific bioassay for opioid kappa-receptor agonists. Eur J Pharmacol 73:235–236
Tao YM, Li QL, Zhang CF, Xu XJ, Chen J, Ju YW, Chi ZQ, Long YQ, Liu JG (2008) LPK-26, a novel kappa-opioid receptor agonist with potent antinociceptive effects and low dependence potential. Eur J Pharmacol 584:306–311
Traynor JR, Guo L, Coop A, Lewis JW, Woods JH (1999) Ring-constrained orvinols as analogs of buprenorphine: differences in opioid activity related to configuration of C(20) hydroxyl group. J Pharmacol Exp Ther 291:1093–1099
Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY (2005) Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther 312:220–230
Acknowledgments
This work was supported by National Natural Science foundation of China (No 30672442), National Great Basic Science Project (2010CB529806); Shanghai Municipal Commission of Education Foundation (No 05BZ14); Innovation Fund for Graduate Students of Fudan University (2006-2007); Shanghai Natural Science Foundation 08ZR1401500; Doctoral Fund of the Ministry of Education of China 20070246088.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hong Qi and Wei Li contribute equally to this work.
Rights and permissions
About this article
Cite this article
Qi, H., Li, W., Qiu, Y. et al. Synthesis and evaluation of κ-opioid receptor agonistic activity and antinociceptive effect of novel morphine analogues, 7α-phenyl-6α,14α-endo-etheno-tetrahydrothebaine with substituted o-, m- and p-amino group. Med Chem Res 20, 1364–1370 (2011). https://doi.org/10.1007/s00044-010-9471-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9471-6